Research progress in applications of LSPR biosensor for clinical medicine detection
-
摘要:
肿瘤标志物在人类医学及恶性肿瘤的早期诊断、治疗监测及预后评估方面具有重要作用。目前血清肿瘤标志物的检测方法主要有放射免疫法(RIA),酶联免疫吸附法(ELISA)及化学发光免疫法(CLEIA)等,这些方法各自存在放射性污染、操作繁琐、检测时间长、价格昂贵等缺点,限制了血清肿瘤标志物在临床医学及肿瘤检测中的应用。新近出现的基于局域表面等离子体共振效应(LSPR)的传感器因在生物医学检测领域极具优势而成为研究热点。基于局域表面等离子体共振效应的生物传感器,利用贵金属纳米结构对周围介质环境变化敏感的基本原理,可将生物分子吸附引发的金属纳米颗粒外界介质折射率的改变转化为可测量的LSPR峰值吸收波长有规律的移动以实现对传感器表面样品的检测,具备检测灵敏度高、特异性好、免标记、设备便携、成本低的优点,具备临床检测潜力。但到目前为止,利用此传感器检测与疾病及肿瘤相关的肿瘤标志物的类似研究报道较少。在本文中,我们针对LSPR生物传感器的传感原理、国内外的研究进展以及我们在此方面的主要研究成果进行了综述。
-
关键词:
- 局域表面等离子体共振效应 /
- 生物传感器 /
- 临床医学 /
- 肿瘤标志物
Abstract:Tumor biomarker plays an important role in early diagnosis, treatment evaluation, and prognosis prediction for human medicine and cancers. At present, the human serum tumor biomarker detection methods mainly include radioimmunoassay (RIA), enzyme linked immunosorbent assay (ELISA), and chemiluminescence immunoassay (CLEIA), which have disadvantages of radioactive contamination, complicated operation, and long detection time and high cost. Various issues exist in these methods which limit its widespread applications in clinic screening. Recently, biosensors based on localized surface plasmon resonance (LSPR) have attracted much research attention for their remarkable superiority in the domain of biomedicine detection. The LSPR biosensor, a novel type of optical fiber-based biosensor, uses an optical fiber or optical fiber bundle to transform biological recognition information into analytically useful signals in the LSPR spectrum, which is suitable for clinical detection because of the advantages of high sensitivity, high specificity, label free, portable equipment and lower cost. However, up to now, there was little progress on the report of the detection of tumor biomarkers associated diseases and tumors by using this LSPR biosensor. In this paper, the principle and research progress of local surface plasmon resonance biosensor, as well as the main findings of our study in the detection of tumor markers are reviewed.
-
Key words:
- localized surface plasmon resonance /
- biosensor /
- clinical medicine /
- tumor biomarker
-

Abstract: Tumor biomarker plays an important role in early diagnosis, treatment evaluation, and prognosis prediction for human medicine and cancers. At present, the human serum tumor biomarker detection methods have defections, such as radioactive contamination, complicated operation, long detection time and high cost, which limit the widespread applications in clinic screening. The LSPR biosensor, a novel type of optical fiber-based biosensor, uses an optical fiber or optical fiber bundle to transform biological recognition information into analytically useful signals in the LSPR spectrum, which is suitable for clinical detection because of the advantages of high sensitivity, high specificity, label free, portable equipment and lower cost. In this paper, the principle and research progress of local surface plasmon resonance biosensor, especially the main findings of our study group, are reviewed.
In this work, the physical characteristics of metal nanostructure were calculated and simulated by discrete dipole approximation (DDA). Combining with reactive ion etching (RIE), nanosphere lithography (NSL) was applied to produce the triangle nanostructures. Eventually, a triangle silver nanostrure array with high refractive index sensitivity was produced. Meanwhile, a primitive ultraviolet-visible spectrometer was constructed to make spectro-analysis. An effective biological sensitive layer was fabricated on the surface of nanostructures by means of 11-mercaptoundecanoic acid (MUA) amine couple method.
To explore the clinical applications of this LSPR biosensor, we firstly used it to detect the microalbuminuria. The anti-human albumin antibody was immobilized on the sensor surface. Different concentrations of commercial albumin and albumin in urine samples from patients were determined according to the peak of LSPR extinction spectra. The biosensor displayed a detection limit of 1 ng/ml and wide dynamic range from 1 ng/ml to 1 mg/ml. Secondly, the anti-HE4 antibody as a probe, which could distinctly recognize HE4, an important ovarian tumor marker, was effectively assembled onto the nanochip surface. Human serum samples were detected and compared using an enzyme-linked immunosorbent assay. The linear range was between 10 pM and 10000 pM, with a detection limit of 4 pM and an excellent correlation between it and enzyme-linked immunosorbent assay. Thirdly, we made a functionalized chip surface with monoclonal anti-SCCa antibodies on the silver nanoparticles for distinct detection of SCCa. Different concentrations of SCCa were successfully tested in both buffer and human serum, with a linear quantitative detection range of 0.1~1000 pM. Lastly, the specific DNA probe was immobilized on the nanochip surface. Wild-type and mutant p53 DNA was detected. The low detection limit was 10 nM, with a wide dynamic range (10 nM~10 μM). Importantly, this sensor could effectively discriminate against single base mutations. By comparing with traditional methods, the advantage of the LSPR biosensor, such as wider detection range, label free, simple operation and short time was clear. It could provide a promising platform for developing clinical diagnostic applications.
-
-
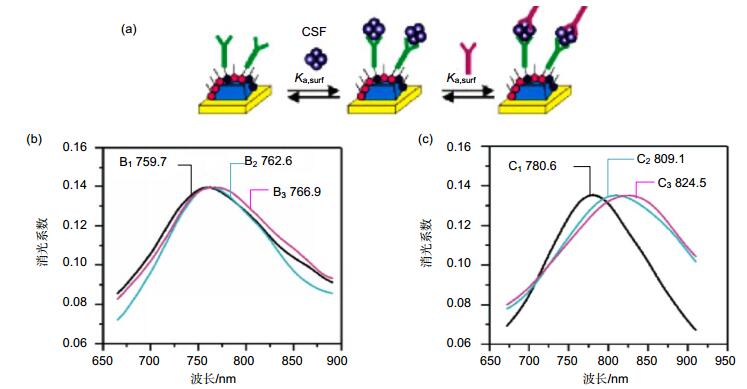
图 2 LSPR纳米传感器检测人脑脊液样本[16]. (a)利用抗体三明治方法检测人脑脊液样本的芯片表面化学反应过程. (b)传感器检测老年人脑脊液样本的波谱图. B1~B3:功能化银纳米加入100 nM anti-ADDL(100 mM EDC活化) (B1),加入老年人脑脊液样本(B2)以及再次加入100 nM anti-ADDL (B3)的波谱图. (c)传感器检测老年患者脑脊液样本的波谱图. C1~C3:功能化银纳米加入100 nM anti-ADDL(100 mM EDC活化) (C1),加入老年人脑脊液样本(C2)以及再次加入100 nM anti-ADDL (C3)的波谱图.
Figure 2. Analysis of human CSF samples using a sandwich assay and the LSPR nanosensor[16]. (a) Surface chemistry for the possible ADDL detection in human CSF samples using the antibody "sandwich"assay. (b) Aging patient: LSPR spectra for each step of the assay. Ag nanoparticles after functionalization with 100 nM anti-ADDL (100 mM EDC) (B1), CSF (B2), 100 nM anti-ADDL (B3). (c) AD patient: LSPR spectra for each step of the assay. Ag nanoparticles after functionalization with 100 nM anti-ADDL (100mM EDC) (C1), CSF(C2), 100 nM anti-ADDL (C3). All measurements were collected in a N2 environment.
图 3 (a) 随机有序圆柱形纳米粒子阵列的离散圆形区域25 mm玻片相机图像. (b)每个圆形区域随机有序纳米圆柱阵列玻片示意图[18].
Figure 3. (a) Camera imaging of a 25 mm glass slide patterned with discrete circular regions of randomly ordered cylindrical nanoparticle arrays using a shadow mask. (b) Schematic showing randomly ordered nano-cylinder arrays within each circular region on the glass slide [18].
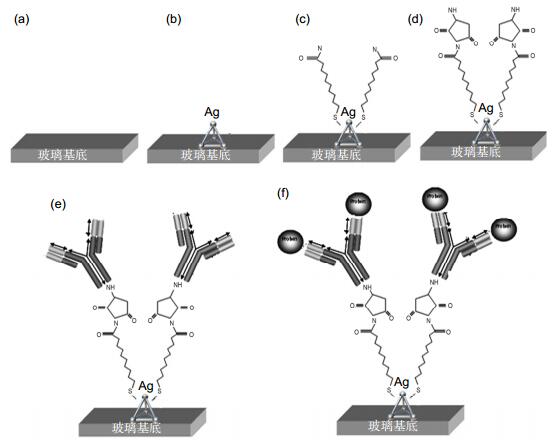
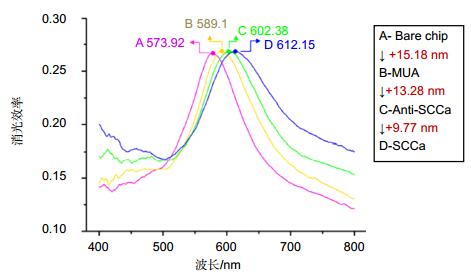
图 4 LSPR纳米传感芯片检测白蛋白过程[26]. (a)芯片玻璃基底. (b)采用NSL技术在芯片表面合成点阵排列的纳米颗粒. (c)纳米表面自主装11-MUA层. (d)孵育EDC和NHS,活化表面巯基. (e)固定白蛋白抗体. (f)不同浓度的标准品和样品与芯片进行免疫分析检测.
Figure 4. Design of LSPR nanobiosensor for albumin detection [26]. (a) Glass substrate. (b) Surface-confined sliver nanoparticles were synthesized using NSL. (c) The nanoparticles were incubated in MUA solution to form a SAM. (d) The substrates were incubated in EDC/NHS. (e) Anti-albumin antibody (10 mg/ml) immobilized the substrate. (f) Varying the concentrations of the albumin or the samples on the substrates completed the albumin immunoassay.
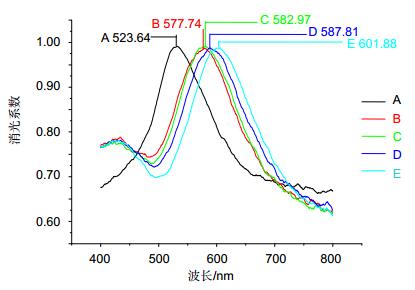
图 6 LSPR传感芯片测定1 μg/ml的白蛋白的波谱图[26]. A:原始基线值. B:巯基化合物在芯片表面形成自组装单分子层(SAM)后的波峰值. C:交联剂作用后的波峰值. D:固定抗白蛋白抗体的波峰值. E: 1 μg/ml白蛋白反应上样波谱图.
Figure 6. Specific LSPR extinction spectrum for 1μg/ml albumin detection [26]. A: Ag nanoparticles before chemical modification. B: MUA (1 mM). C: EDC/NHS solution. D: antibody (10 mg/ml). E: albumin (1 mg/ml). All spectra were collected in air.
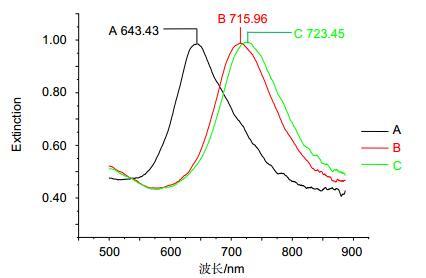
图 8 LSPR传感器检测100 pM SCCA的波谱图[31]. A:原始基线值. B: MUA在芯片表面形成SAM后的波峰值. C:固定单克隆抗体的波峰值. D: 100 pM SCCA反应点样后的波谱图.
Figure 8. Specific LSPR extinction spectrum for 100 pM SCCA detection [31]. A: Bare silver nanochip. B: Modification of 1 mM MUA. C: Incubation with 10 μg/mL monoclonal anti-SCCa. D: Detection of 100 pM SCCa.
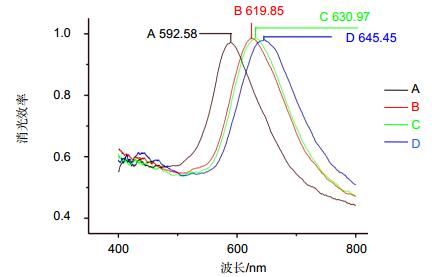
图 9 LSPR传感芯片测定HE4的波谱图[36]. A:原始基线值. B: 11-MUA自组装单分子层. C:固定10 μg/ml抗HE4单克隆抗体. D:与500 pM的HE4标准品进行抗原抗体反应(Δλmax+14.48 nm).
Figure 9. Specific LSPR extinction spectrum for HE4 detection [36]. A: Bare silver nanochip. B: 1 mM 11-mercaptoundecanoic acid. C: functionalized biosensor with 10 μg/mL antibody. D: 500 pM HE4.
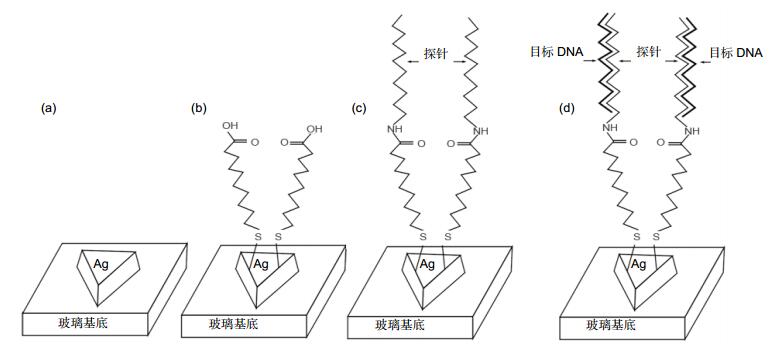
图 10 构建检测p53基因突变的LSPR纳米生物传感器[43]. (a)利用NSL方法在玻璃基底上制备三角柱银纳米. (b)在银纳米表面修饰MUA形成自组装层. (c) EDC/NHS催化下将末端修饰氨基的DNA探针固定在纳米表面. (d)固定探针与目标序列杂交.
Figure 10. (a) Triangular Ag nanoparticles were fabricated on a glass substrate using NSL. (b) The nanoparticles were modified with MUA to form a SAM. (c) Amine-terminated DNA probes were immobilized on the MUA-coated nanoparticles with EDC/NHS activation. (d) Target DNA was hybridized with the immobilized probe.
-
[1] Endo T, Kerman K, Nagatani N, et al. Multiple label-free detection of antigen-antibody reaction using localized surface plasmon resonance-based core shell structured nanoparticle layer nanochip[J]. Analytical Chemistry, 2006, 78(18): 6465-6475. doi: 10.1021/ac0608321
[2] Cantale V, Simeone F C, Gambari R, et al. Gold nano-islands on FTO as plasmonic nanostructures for biosensors[J]. Sensors and Actuators B: Chemical, 2011, 152(2): 206-213. doi: 10.1016/j.snb.2010.12.008
[3] Haynes C L, Van Duyne R P. Nanosphere lithography: a versatile nanofabrication tool for studies of size-dependent nanoparticle optics[J]. The Journal of Physical Chemistry B, 2001, 105(24): 5599-5611. doi: 10.1021/jp010657m
[4] Haes A J, Van Duyne R P. A unified view of propagating and localized surface plasmon resonance biosensors[J]. Analytical and Bioanalytical Chemistry, 2004, 379(7-8): 920-930. doi: 10.1007/s00216-004-2708-9
[5] Willets K A, Van Duyne R P. Localized surface plasmon resonance spectroscopy and sensing[J]. Annual Review of Physical Chemistry, 2007, 58(1): 267-297. doi: 10.1146/annurev.physchem.58.032806.104607
[6] Hutter E, Fendler J H. Exploitation of localized surface plasmon resonance[J]. Advanced Materials, 2004, 16(19): 1685-1706. doi: 10.1002/(ISSN)1521-4095
[7] Haes A J, Van Duyne R P. A nanoscale optical biosensor: sensitivity and selectivity of an approach based on the localized surface Plasmon resonance spectroscopy of triangular silver nanoparticles[J]. Journal of the American Chemical Society, 2002, 124(35): 10596-10604. doi: 10.1021/ja020393x
[8] Englebienne P. Use of colloidal gold surface plasmon resonance peak shift to infer affinity constants from the interactions between protein antigens and antibodies specific for single or multiple epitopes[J]. The Analyst, 1998, 123(7): 1599-1603. doi: 10.1039/a804010i
[9] Kreuzer M P, Quidant R, Salvador J P, et al. Colloidal-based localized surface plasmon resonance (LSPR) biosensor for the quantitative determination of stanozolol[J]. Analsytical and Bioanalytical Chemistry, 2008, 391(5): 1813-1820. doi: 10.1007/s00216-008-2022-z
[10] Grabar K C, Freeman R G, Hommer M B, et al. Preparation and Characterization of Au Colloid Monolayers[J]. Analytical Chemistry, 1995, 67(4): 735-743. doi: 10.1021/ac00100a008
[11] Nath N, Chilkoti A. A colorimetric gold nanoparticle sensor to interrogate biomolecular interactions in real time on a surface[J]. Analytical Chemistry, 2002, 74(3): 504-509. doi: 10.1021/ac015657x
[12] Sherry Leif J, Jin Rongchao, Mirkin Chad A, et al. Localized surface Plasmon resonance spectroscopy of single silver triangular nanoprisms[J]. Nano Letters, 2006, 6(9): 2060-2065. doi: 10.1021/nl061286u
[13] Chan George H, Zhao Jing, Hicks Erin M, et al. Plasmonic properties of copper nanoparticles fabricated by nanosphere lithography[J]. Nano Letters, 2007, 7(7): 1947-1952. doi: 10.1021/nl070648a
[14] Chan George H, Zhao Jing, Schatz George C, et al. Localized surface Plasmon resonance spectroscopy of triangular aluminum nanoparticles[J]. The Journal of Physical Chemistry C, 2008, 112(36): 13958-13963. doi: 10.1021/jp804088z
[15] Haes A J, Van Duyne R P. Nanoscale optical biosensors based on localized surface plasmon resonance spectroscopy[J]. Proceedings of SPIE, 2003, 5221: 47-58. doi: 10.1117/12.508308
[16] Haes Amanda, Chang Lei, Klein William L, et al. Detection of a biomarker for Alzheimer's disease from synthetic and clinical samples using a nanoscale optical biosensor[J]. Journal of the American Chemical Society, 2005, 127(7): 2264-2271. doi: 10.1021/ja044087q
[17] Riboh J C, Haes A J, McFarland A D, et al. A nanoscale optical biosensor: real-time immunoassay in physiological buffer enabled by improved nanoparticle adhesion[J]. The Journal of Physical Chemistry B, 2003, 107(8): 1772-1780. doi: 10.1021/jp022130v
[18] Ruemmele J A, Hall W P, Ruvuna L K, et al. A localized surface Plasmon resonance imaging instrument for multiplexed biosensing[J]. Analytical Chemistry, 2013, 85(9): 4560-4566. doi: 10.1021/ac400192f
[19] Truong Phuoc Long, Cao Cuong, Park Sungho, et al. A new method for non-labeling attomolar detection of diseases based on an individual gold nanorod immunosensor[J]. Lab on a Chip, 2011, 11(15): 2591-2597. doi: 10.1039/c1lc20085b
[20] Yamamichi J, Ojima T, Yurugi K, et al. Single-step, label-free quantification of antibody in human serum for clinical applications based on localized surface plasmon resonance[J]. Nanomedicine, 2011, 7(6): 889-895. doi: 10.1016/j.nano.2011.02.002
[21] Lee Jin Ho, Kim Byung chan, Oh Byung Keum, et al. Highly sensitive localized surface plasmon resonance immunosensor for label-free detection of HIV-1[J]. Nanomedicine, 2013, 9(7): 1018-1026. doi: 10.1016/j.nano.2013.03.005
[22] Yoo S Y, Kim D K, Park T J, et al. Detection of the most common corneal dystrophies caused by BIGH3 gene point mutations using a multispot gold-capped nanoparticle array chip[J]. Analytical Chemistry, 2010, 82(4): 1349-1357. doi: 10.1021/ac902410z
[23] Tang Liang, Casas Justin. Quantification of cardiac biomarkers using label-free and multiplexed gold nanorod bioprobes for myocardial infarction diagnosis[J]. Biosensors and Bioelectronics, 2014, 61: 70-75. doi: 10.1016/j.bios.2014.04.043
[24] Lin Hsing Ying, Huang C S, Liu Yu Chia, et al. Multiplex fiber-optic biosensor using multiple particle Plasmon resonances[J]. Proceedings of SPIE, 2012, 8351: 83512S. https://www.spiedigitallibrary.org/redirect/proceedings/proceeding?doi=10.1117/12.914383
[25] Aćimović S S, Ortega M A, Sanz V, et al. LSPR chip for parallel, rapid and sensitive detection of cancer markers in serum[J]. Nano Letters, 2014, 14(5): 2636-2641. doi: 10.1021/nl500574n
[26] Lai Ting, Hou Qiannan, Yang Huan, et al. Clinical application of a novel sliver nanoparticles biosensor based on localized surface Plasmon resonance for detecting the microalbuminuria[J]. Acta Biochimica et Biophysica Sinica, 2010, 42(11): 787-792. doi: 10.1093/abbs/gmq085
[27] Ferenczy A, Franco E. Persistent human papillomavirus infection and cervical neoplasia[J]. The Lancet Oncology, 2002, 3(1): 11-16. doi: 10.1016/S1470-2045(01)00617-9
[28] Parkin D M, Bray F, Ferlay J, et al. Global cancer statistics 2002[J]. CA: A Cancer Journal for Clinicians, 2005, 55(2): 74-108. doi: 10.3322/canjclin.55.2.74
[29] Gadducci A, Tana R, Cosio S, et al. The serum assay of tumour markers in the prognostic evaluation, treatment monitoring and follow-up of patients with cervical cancer: a review of the literature[J]. Critical Reviews in Oncology/Hematology, 2008, 66(1): 10-20. doi: 10.1016/j.critrevonc.2007.09.002
[30] Esajas M D, Duk J M, de Bruijn H W A, et al. Clinical value of routine serum squamous cell carcinoma antigen in follow-up of patients with early-stage cervSical cancer[J]. Journal of Clinical Oncology, 2001, 19(19): 3960-3966. doi: 10.1200/JCO.2001.19.19.3960
[31] Zhao Qianying, Duan Ruiqi, Yuan Jialing, et al. A reusable localized surface plasmon resonance biosensor for quantitative detection of serum squamous cell carcinoma antigen in cervical cancer patients based on silver nanoparticles array[J]. International Journal of Nanomedicine, 2014, 9: 1097-1104. https://www.ncbi.nlm.nih.gov/pubmed/24591830
[32] Shah C A, Lowe K A, Paley P, et al. Influence of ovarian cancer risk status on the diagnostic performance of the serum biomarkers mesothelin, HE4, and CA125[J]. Cancer Epidemiology Biomarkers & Prevention, 2009, 18(5): 1365-1372. https://www.ncbi.nlm.nih.gov/pmc/articles/instance/2714056/
[33] Hellstrom I, Heagerty P J, Swisher E M, et al. Detection of the HE4 protein in urine as a biomarker for ovarian neoplasms[J]. Cancer Letters, 2010, 296(1): 43-48. doi: 10.1016/j.canlet.2010.03.013
[34] Anastasi E, Giovanna M G, Viggiani V, et al. HE4: a new potential early biomarker for the recurrence of ovarian cancer[J]. Tumor Biology, 2010, 31(2): 113-119. doi: 10.1007/s13277-009-0015-y
[35] Yurkovebtsky Z, Skates S, Lomakin A, et al. Development of a multimarker assay for early detection of ovarian cancer[J]. Journal of Clinical Oncology, 2010, 28(13): 2159-2166. doi: 10.1200/JCO.2008.19.2484
[36] Yuan Jialing, Duan Ruiqi, Yang Huan, et al. Detection of serum human epididymis secretory protein 4 in patients with ovarian cancer using a label-free biosensor based on localized surface plasmon resonance[J]. International Journal of Nanomedicine, 2012, 7: 2921-2928. http://www.oalib.com/paper/1357668
[37] Yamaguchi A, Kurosaka Y, Fushida S, et al. Expression of p53 protein in colorectal cancer and its relationship to short-term prognosis[J]. Cancer, 1992, 70(12): 2778-2784. doi: 10.1002/(ISSN)1097-0142
[38] Flamini G, Curigliano G, Ratto C, et al. Prognostic significance of cytoplasmic p53 overexpression in colorectal cancer. An immunohistochemical analysis[J]. European Journal of Cancer, 1996, 32(5): 802-806. doi: 10.1016/0959-8049(95)00625-7
[39] Gu Jian, Zhang Lidong, Swisher Stephen G, et al. Induction of p53-regulated genes in lung cancer cells: implications of the mechanism for adenoviral p53-mediated apoptosis[J]. Oncogene, 2004, 23(6): 1300-1307. doi: 10.1038/sj.onc.1207239
[40] Mazars R, Pujol P, Maudelonde T, et al. P53 mutations in ovarian cancer: a late event[J]. Oncogene, 1991, 6(9): 1685-1690. https://www.researchgate.net/publication/21231522_p53_mutations_in_ovarian_cancer_A_late_event
[41] Shahin M S, Hughes J H, Sood A K, et al. The Prognostic significance of p53 tumor suppressor gene alterations in ovarian carcinoma[J]. Cancer, 2000, 89(9): 2006-2017. doi: 10.1002/1097-0142(20001101)89:9<2006::AID-CNCR18>3.3.CO;2-Z
[42] Sagarra R A M, Andrade L A L A, Martinez E Z, et al. P53 and Bcl-2 as prognostic predictors in epithelial ovarian cancer[J]. International Journal of Gynecological Cancer, 2002, 12(6): 720-727. doi: 10.1046/j.1525-1438.2002.01135.x
[43] Duan Ruiqi, Yuan Jialing, Yang Huan, et al. Detection of p53 gene mutation by using a novel biosensor based on localized surface Plasmon resonance[J]. Neoplasma, 2012, 59(3): 348-353. doi: 10.4149/neo_2012_045
-

 E-mail Alert
E-mail Alert RSS
RSS

 下载:
下载: