| Citation: |
|
Water-sensitive multicolor luminescence in lanthanide-organic framework for anti-counterfeiting
-
Abstract
The development of high-level anti-counterfeiting techniques is of great significance in economics and security issues. However, intricate reading methods are required to obtain multi-level information stored in different colors, which greatly limits the application of anti-counterfeiting technology on solving real world problems. Herein, we realize multicolor information anti-counterfeiting under simply external stimulation by utilizing the functional groups and multiple emission centers of lanthanide metal organic framework (Ln-MOFs) to tune luminescence color. Water responsive multicolor luminescence represented by both the tunable color from red to blue within the visible region and high sensitive responsivity has been achieved, owing to the increased nonradiative decay pathways and enhanced Eu3+-to-ligand energy back transfer. Remarkably, information hidden in different colors needs to be read with a specific water content, which can be used as an encryption key to ensure the security of the information for high-level anti-counterfeiting. -
-
References
[1] Ren W, Lin GG, Clarke C, Zhou JJ, Jin DY. Optical nanomaterials and enabling technologies for high-security-level anticounterfeiting. Adv Mater 32, 1901430 (2020). doi: 10.1002/adma.201901430 [2] Staake T, Thiesse F, Fleisch E. The emergence of counterfeit trade: a literature review. Eur J Mark 43, 320–349 (2009). doi: 10.1108/03090560910935451 [3] Li RM, Zhang YT, Tan J, Wan JX, Guo J et al. Dual-mode encoded magnetic composite microsphere based on fluorescence reporters and raman probes as covert tag for anticounterfeiting applications. ACS Appl Mater Interfaces 8, 9384–9394 (2016). doi: 10.1021/acsami.6b02359 [4] Prime EL, Solomon DH. Australia’s plastic banknotes: fighting counterfeit currency. Angew Chem Int Ed 49, 3726–3736 (2010). doi: 10.1002/anie.200904538 [5] Yao WJ, Tian QY, Wu W. Tunable emissions of upconversion fluorescence for security applications. Adv Opt Mater 7, 1801171 (2019). doi: 10.1002/adom.201801171 [6] Ji XF, Wu RT, Long LL, Ke XS, Guo CX et al. Encoding, reading, and transforming information using multifluorescent supramolecular polymeric hydrogels. Adv Mater 30, 1705480 (2018). doi: 10.1002/adma.201705480 [7] Arppe R, Sørensen TJ. Physical unclonable functions generated through chemical methods for anti-counterfeiting. Nat Rev Chem 1, 0031 (2017). doi: 10.1038/s41570-017-0031 [8] Zhang C, Yang L, Zhao J, Liu BH, Han MY et al. White‐light emission from an integrated upconversion nanostructure: toward multicolor displays modulated by laser power. Angew Chem Int Ed 54, 11531–11535 (2015). doi: 10.1002/anie.201504518 [9] Lu YQ, Zhao JB, Zhang R, Liu YJ, Liu DM et al. Tunable lifetime multiplexing using luminescent nanocrystals. Nat Photonics 8, 32–36 (2014). doi: 10.1038/nphoton.2013.322 [10] Chen GY, Damasco J, Qiu HL, Shao W, Ohulchanskyy TY et al. Energy-cascaded upconversion in an organic dye-sensitized core/shell fluoride nanocrystal. Nano Lett 15, 7400–7407 (2015). doi: 10.1021/acs.nanolett.5b02830 [11] Zhou JJ, Wen SH, Liao JY, Clarke C, Tawfik SA et al. Activation of the surface dark-layer to enhance upconversion in a thermal field. Nat Photonics 12, 154–158 (2018). doi: 10.1038/s41566-018-0108-5 [12] Zhang JC, Pan C, Zhu YF, Zhao LZ, He HW et al. Achieving thermo-mechano-opto-responsive bitemporal colorful luminescence via multiplexing of dual lanthanides in piezoelectric particles and its multidimensional anticounterfeiting. Adv Mater 30, 1804644 (2018). doi: 10.1002/adma.201804644 [13] Cai GR, Jiang HL. A modulator-induced defect-formation strategy to hierarchically porous metal-organic frameworks with high stability. Angew Chem Int Ed 56, 563–567 (2017). doi: 10.1002/anie.201610914 [14] Chen ZJ, Li PH, Anderson R, Wang XJ, Zhang X et al. Balancing volumetric and gravimetric uptake in highly porous materials for clean energy. Science 368, 297–303 (2020). doi: 10.1126/science.aaz8881 [15] Islamoglu T, Chen ZJ, Wasson MC, Buru CT, Kirlikovali KO et al. Metal-organic frameworks against toxic chemicals. Chem Rev 120, 8130–8160 (2020). doi: 10.1021/acs.chemrev.9b00828 [16] Lee S, Kapustin EA, Yaghi OM. Coordinative alignment of molecules in chiral metal-organic frameworks. Science 353, 808–811 (2016). doi: 10.1126/science.aaf9135 [17] Li J, Wang XX, Zhao GX, Chen CL, Chai ZF et al. Metal-organic framework-based materials: superior adsorbents for the capture of toxic and radioactive metal ions. Chem Soc Rev 47, 2322–2356 (2018). doi: 10.1039/C7CS00543A [18] Li P, Vermeulen NA, Malliakas CD, Gómez-Gualdrón DA, Howarth AJ et al. Bottom-up construction of a superstructure in a porous uranium-organic crystal. Science 356, 624–627 (2017). doi: 10.1126/science.aam7851 [19] Wang B, Zhang X, Huang HL, Zhang ZJ, Yildirim T et al. A microporous aluminum-based metal-organic framework for high methane, hydrogen, and carbon dioxide storage. Nano Res 14, 507–511 (2021). doi: 10.1007/s12274-020-2713-0 [20] Yu BX, Ye G, Chen J, Ma SQ. Membrane-supported 1D MOF hollow superstructure array prepared by polydopamine-regulated contra-diffusion synthesis for uranium entrapment. Environ Pollut 253, 39–48 (2019). doi: 10.1016/j.envpol.2019.06.114 [21] Zhang X, Lin RB, Wang J, Wang B, Liang B et al. Optimization of the pore structures of MOFs for record high hydrogen volumetric working capacity. Adv Mater 32, 1907995 (2020). doi: 10.1002/adma.201907995 [22] Yuan HY, Tao JF, Li NX, Karmakar A, Tang CH et al. On‐chip tailorability of capacitive gas sensors integrated with metal-organic framework films. Angew Chem Int Ed 58, 14089–14094 (2019). doi: 10.1002/anie.201906222 [23] Yao YN, Gao ZH, Lv YC, Lin XQ, Liu YY et al. Heteroepitaxial growth of multiblock Ln-MOF microrods for photonic barcodes. Angew Chem Int Ed 58, 13803–13807 (2019). doi: 10.1002/anie.201907433 [24] Kim H, Yang S, Rao SR, Narayanan S, Kapustin EA et al. Water harvesting from air with metal-organic frameworks powered by natural sunlight. Science 356, 430–434 (2017). doi: 10.1126/science.aam8743 [25] Nguyen HL, Hanikel N, Lyle SJ, Zhu CH, Proserpio DM et al. A porous covalent organic framework with voided square grid topology for atmospheric water harvesting. J Am Chem Soc 142, 2218–2221 (2020). doi: 10.1021/jacs.9b13094 [26] Ma D, Li P, Duan XY, Li JZ, Shao PP et al. A hydrolytically stable vanadium(IV) metal-organic framework with photocatalytic bacteriostatic activity for autonomous indoor humidity control. Angew Chem Int Ed 59, 3905–3909 (2020). doi: 10.1002/anie.201914762 [27] Burtch NC, Jasuja H, Walton KS. Water stability and adsorption in metal-organic frameworks. Chem Rev 114, 10575–10612 (2014). doi: 10.1021/cr5002589 [28] Hao JN, Li YS. Concurrent modulation of competitive mechanisms to design stimuli-responsive Ln-MOFs: a light-operated dual-mode assay for oxidative DNA damage. Adv Funct Mater 29, 1903058 (2019). doi: 10.1002/adfm.201903058 [29] Zhao NS, Li LJ, Song XZ, Zhu M, Hao ZM et al. Lanthanide ion codoped emitters for tailoring emission trajectory and temperature sensing. Adv Funct Mater 25, 1463–1469 (2015). doi: 10.1002/adfm.201402061 [30] Li ZQ, Wang GN, Ye YX, Li B, Li HR et al. Loading photochromic molecules into a luminescent metal-organic framework for information anticounterfeiting. Angew Chem Int Edit 58, 18025–18031 (2019). doi: 10.1002/anie.201910467 [31] Razavi SAA, Morsali A. Linker functionalized metal-organic frameworks. Coordin Chem Rev 388, 213023 (2019). [32] Guillerm V, Weseliński ŁJ, Belmabkhout Y, Cairns AJ, D'Elia V et al. Discovery and introduction of a (3, 18)-connected net as an ideal blueprint for the design of metal-organic frameworks. Nat Chem 6, 673–680 (2014). doi: 10.1038/nchem.1982 [33] Xue DX, Belmabkhout Y, Shekhah O, Jiang H, Adil K et al. Tunable rare earth fcu-MOF platform: access to adsorption kinetics driven gas/vapor separations via pore size contraction. J Am Chem Soc 137, 5034–5040 (2015). doi: 10.1021/ja5131403 [34] Yu Y, Ma JP, Dong YB. Luminescent humidity sensors based on porous Ln3+-MOFs. CrystEngComm 14, 7157–7160 (2012). doi: 10.1039/c2ce26210j [35] Yu L, Zheng QT, Wang H, Liu CX, Huang XQ et al. Double-color lanthanide metal-organic framework based logic device and visual ratiometric fluorescence water microsensor for solid pharmaceuticals. Anal Chem 92, 1402–1408 (2020). doi: 10.1021/acs.analchem.9b04575 [36] Li L, Zhu YL, Zhou XH, Brites CDS, Ananias D et al. Visible‐light excited luminescent thermometer based on single lanthanide organic frameworks. Adv Funct Mater 26, 8677–8684 (2016). doi: 10.1002/adfm.201603179 [37] Heine J, Müller-Buschbaum K. Engineering metal-based luminescence in coordination polymers and metal-organic frameworks. Chem Soc Rev 42, 9232–9242 (2013). doi: 10.1039/c3cs60232j -
Supplementary Information
Supplementary information for Water-sensitive multicolor luminescence in lanthanide-organic framework for anti-counterfeiting 
-
Access History
Article Metrics
-
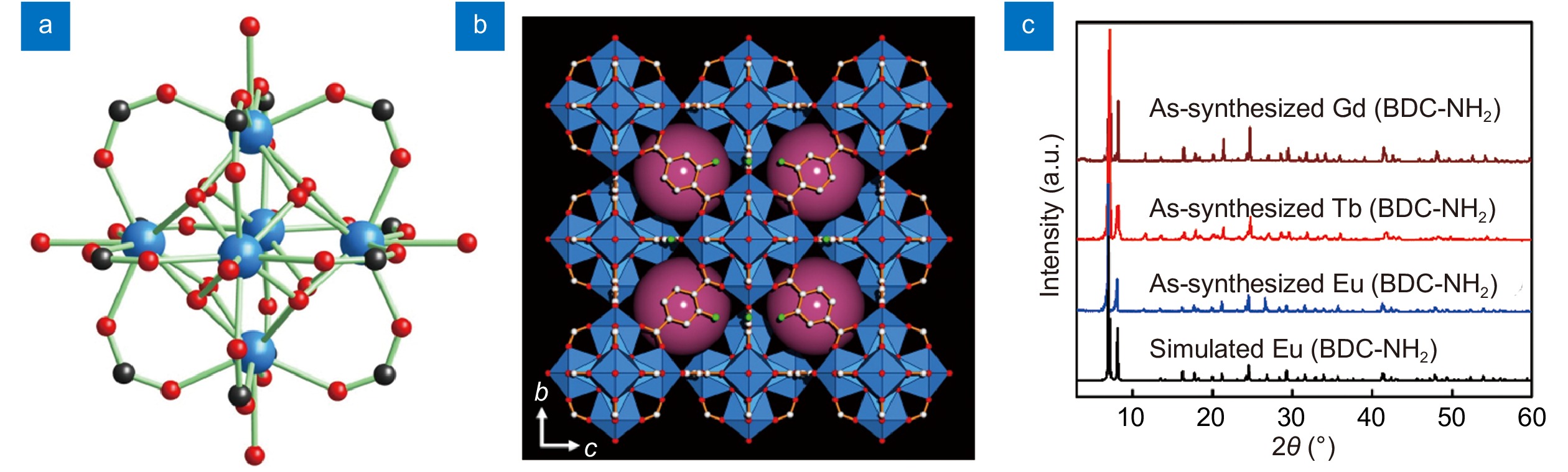
Figure 1.
(a) Structure of 12-connected hexanuclear Eu cluster [Eu6(μ3-OH)8(CO2)12]. Eu, blue; C, black; O, red. (b) Crystal structure of Eu(BDC-NH2) viewed along the a axis. Eu, blue polyhedra; C, gray; O, red; N, blue; H atoms, Me2NH2 cations and free DMF molecules are omitted for clarity. (c) Powder X-ray diffraction patterns of Eu(BDC-NH2), Tb(BDC-NH2) and Gd(BDC-NH2).
-
Figure 2.
(a) The concentration of dissolved ligand H2BDC-NH2 in Eu(BDC-NH2) suspension at different time. (b) N2 sorption isotherms of Eu(BDC-NH2) before (red) and after (blue) being treated with water at 77 K. Solid symbols: adsorption, open symbols: desorption.
-
Figure 3.
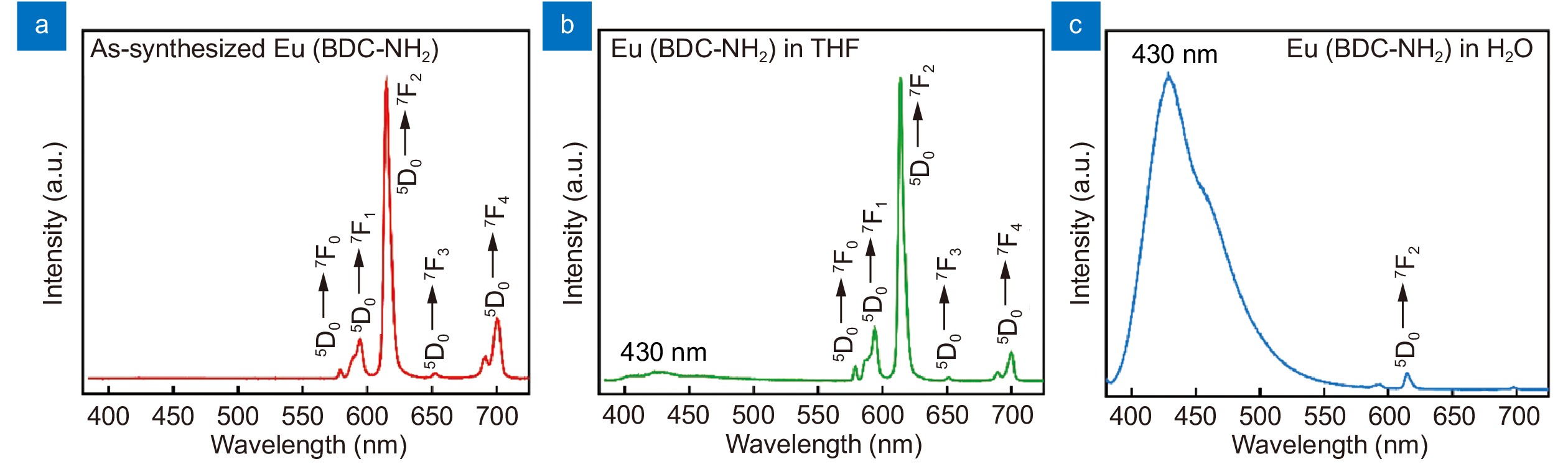
Emission spectra of Eu(BDC-NH2) in the solid state (a) suspended in THF (b) and in water (c) excited at 375 nm.
-
Figure 4.
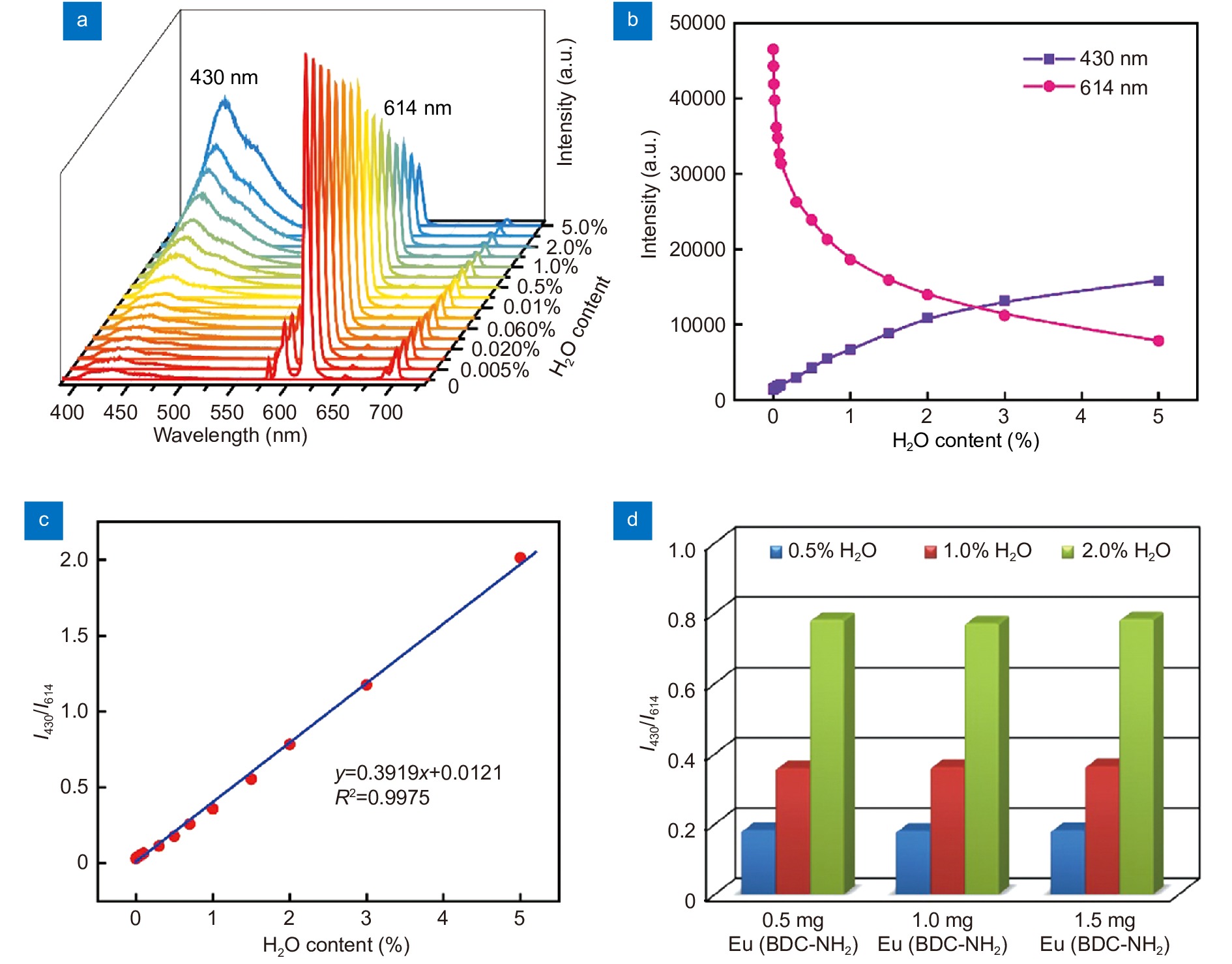
(a) Emission spectra of Eu(BDC-NH2) suspended in THF with different water content excited at 375 nm. (b) Intensities of ligand luminescence and 5D0 → 7F2 transition of Eu(BDC-NH2) in the presence of various content of water. (c) The relationship of luminescence intensity ratio (I430/I614) and water content. (d) Luminescence intensity ratio (I430/I614) measured in THF/H2O mixture with given water content (0.5, 1 and 2 vol%) using different amounts of Eu(BDC-NH2) (0.5, 1.0 and 1.5 mg).
-
Figure 5.
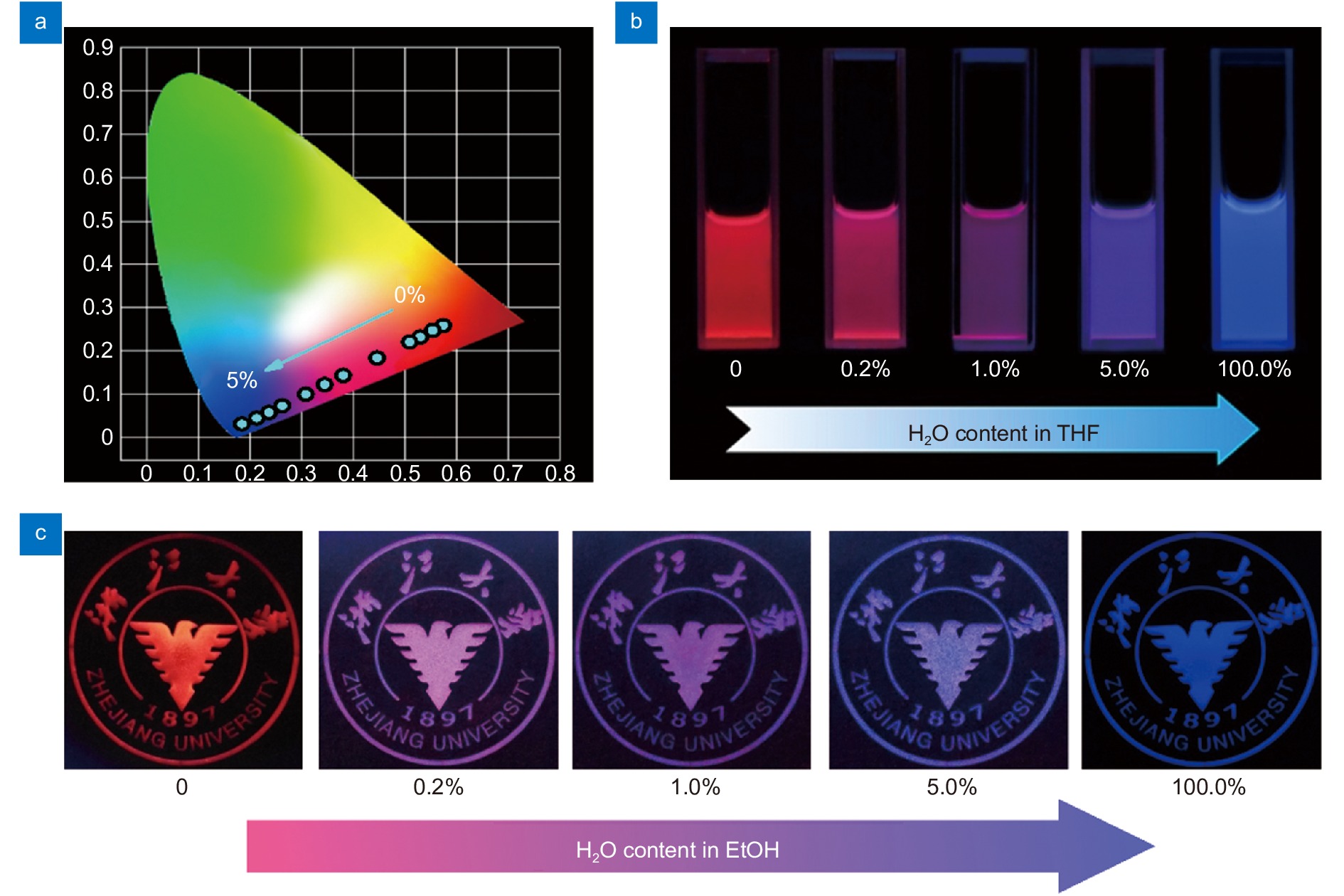
(a) CIE chromaticity coordinates of the luminescence color of Eu(BDC-NH2) in THF with different water content (c = 0, 0.01, 0.04, 0.1, 0.3, 0.5, 0.7, 1, 1.5, 2, 3, and 5 vol%, respectively). Photographs of Eu(BDC-NH2) in (b) THF and (c) EtOH with different water content excited at 365 nm.
-
Figure 6.
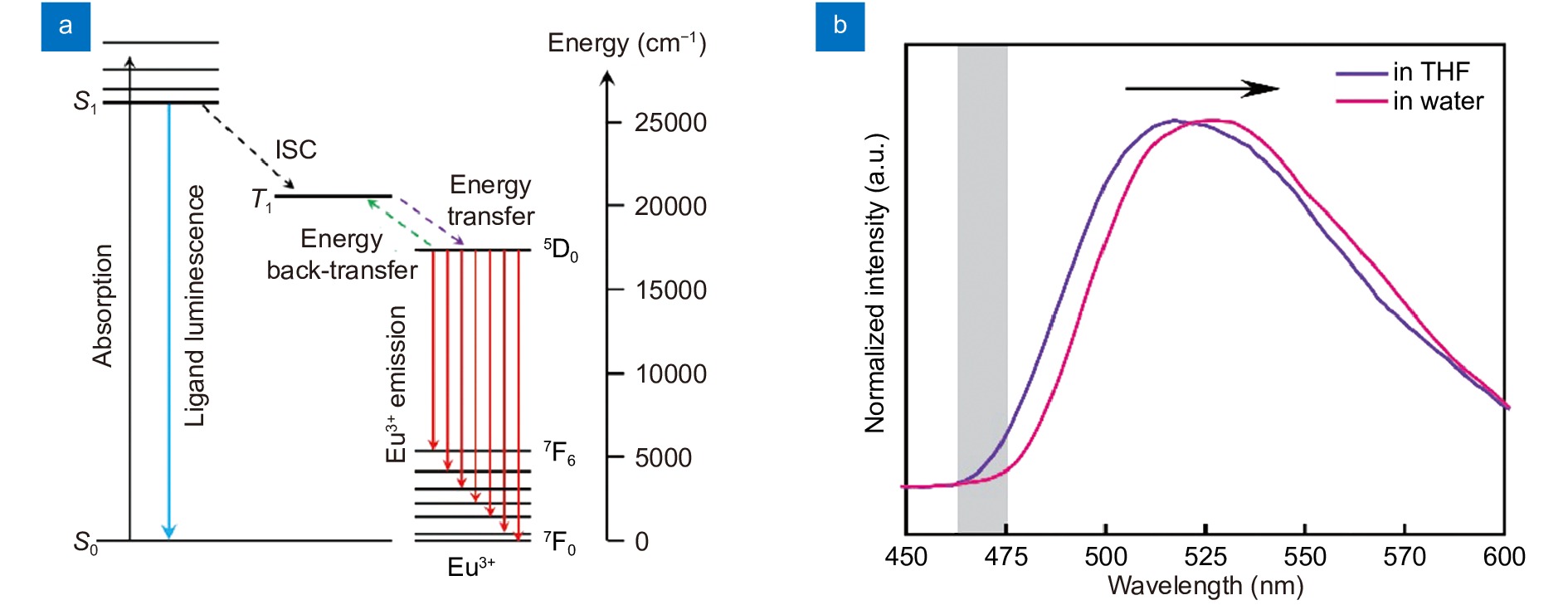
(a) Schematic representation of energy transfer process in Eu(BDC-NH2). (b) Phosphorescence spectra of Gd(BDC-NH2) at 77 K excited at 375 nm in frozen THF and water.
- Figure FIG. 825..

 E-mail Alert
E-mail Alert RSS
RSS

 DownLoad:
DownLoad: