| Citation: | Sun N, Gan Y, Wu YJ et al. Single-beam optical trap-based surface-enhanced raman scattering optofluidic molecular fingerprint spectroscopy detection system. Opto-Electron Adv 8, 240182 (2025). doi: 10.29026/oea.2025.240182 |
Single-beam optical trap-based surface-enhanced raman scattering optofluidic molecular fingerprint spectroscopy detection system
-
Abstract
In this study, we developed a single-beam optical trap-based surface-enhanced Raman scattering (SERS) optofluidic molecular fingerprint spectroscopy detection system. This system utilizes a single-beam optical trap to concentrate free silver nanoparticles (AgNPs) within an optofluidic chip, significantly enhancing SERS performance. We investigated the optical field distribution characteristics within the tapered fiber using COMSOL simulation software and established a MATLAB simulation model to validate the single-beam optical trap's effectiveness in capturing AgNPs, demonstrating the theoretical feasibility of our approach. To verify the particle capture efficacy of the system, we experimentally controlled the optical trap's on-off state to manage the capture and release of particles precisely. The experimental results indicated that the Raman signal intensity in the capture state was significantly higher than in the non-capture state, confirming that the single-beam optical trap effectively enhances the SERS detection capability of the optofluidic detection system. Furthermore, we employed Raman mapping techniques to investigate the impact of the capture area on the SERS effect, revealing that the spectral intensity of molecular fingerprints in the laser-trapping region is significantly improved. We successfully detected the Raman spectrum of crystal violet at a concentration of 10−9 mol/L and pesticide thiram at a concentration of 10−5 mol/L, further demonstrating the ability of the single-beam optical trap in enhancing the molecular fingerprint spectrum identification capability of the SERS optofluidic chips. The optical trapping SERS optofluidic detection system developed in this study, as a key component of an integrated optoelectronic sensing system, holds the potential for integration with portable high-power lasers and high-performance Raman spectrometers. This integration is expected to advance highly integrated technologies and significantly enhance the overall performance and portability of optoelectronic sensing systems. -
-
References
[1] Li GQ, Zhao X, Tang X et al. Wearable hydrogel SERS chip utilizing plasmonic trimers for uric acid analysis in sweat. Nano Lett 24, 13447–13454 (2024). doi: 10.1021/acs.nanolett.4c04267 [2] Bharati MSS, Soma VR. Flexible SERS substrates for hazardous materials detection: recent advances. Opto-Electron Adv 4, 210048 (2021). doi: 10.29026/oea.2021.210048 [3] Shao MR, Ji C, Tan JB et al. Ferroelectrically modulate the Fermi level of graphene oxide to enhance SERS response. Opto-Electron Adv 6, 230094 (2023). doi: 10.29026/oea.2023.230094 [4] Wang ZK, Sha HY, Zhu Y et al. A compact device of optical fiber taper coupled monolayer silver nanoparticles for Raman enhancement. J Lightwave Technol 42, 865–874 (2024). doi: 10.1109/JLT.2023.3317671 [5] Lin CL, Li YY, Peng YS, et al. Recent development of surface-enhanced Raman scattering for biosensing. J Nanobiotechnol 21, 149 (2023). doi: 10.1186/s12951-023-01890-7 [6] Zhang J, Zhang XL, Chen SM et al. Surface-enhanced Raman scattering properties of multi-walled carbon nanotubes arrays-Ag nanoparticles. Carbon 100, 395–407 (2016). doi: 10.1016/j.carbon.2016.01.025 [7] Kim K, Lee H, Choi J et al. Silver-coated dye-embedded silica beads: a core material of dual tagging sensors based on fluorescence and Raman scattering. ACS Appl Mater Interfaces 3, 324–330 (2011). doi: 10.1021/am1009474 [8] Chen JM, Huang YJ, Kannan P et al. Flexible and adhesive surface enhance Raman scattering active tape for rapid detection of pesticide residues in fruits and vegetables. Anal Chem 88, 2149–2155 (2016). doi: 10.1021/acs.analchem.5b03735 [9] He LL, Chen T, Labuza TP. Recovery and quantitative detection of thiabendazole on apples using a surface swab capture method followed by surface-enhanced Raman spectroscopy. Food Chem 148, 42–46 (2014). doi: 10.1016/j.foodchem.2013.10.023 [10] Yuan JP, Lai YC, Duan JL et al. Synthesis of a β-cyclodextrin-modified Ag film by the galvanic displacement on copper foil for SERS detection of PCBs. J Colloid Interface Sci 365, 122–126 (2012). doi: 10.1016/j.jcis.2011.08.075 [11] Li JF, Huang YF, Ding Y et al. Shell-isolated nanoparticle-enhanced Raman spectroscopy. Nature 464, 392–395 (2010). doi: 10.1038/nature08907 [12] Muro CK, Doty KC, Bueno J et al. Vibrational spectroscopy: recent developments to revolutionize forensic science. Anal Chem 87, 306–327 (2015). doi: 10.1021/ac504068a [13] Whitesides GM. The origins and the future of microfluidics. Nature 442, 368–373 (2006). doi: 10.1038/nature05058 [14] Yager P, Edwards T, Fu E et al. Microfluidic diagnostic technologies for global public health. Nature 442, 412–418 (2006). doi: 10.1038/nature05064 [15] Yuan XL, Darcie T, Wei ZY et al. Microchip imaging cytometer: making healthcare available, accessible, and affordable. Opto-Electron Adv 5, 210130 (2022). doi: 10.29026/oea.2022.210130 [16] Wang MM, Tu E, Raymond DE et al. Microfluidic sorting of mammalian cells by optical force switching. Nat Biotechnol 23, 83–87 (2005). doi: 10.1038/nbt1050 [17] Zhu JM, Zhu XQ, Zuo YF et al. Optofluidics: the interaction between light and flowing liquids in integrated devices. Opto-Electron Adv 2, 190007 (2019). [18] Monisha K, Suresh K, Bankapur A et al. Optically printed plasmonic fiber tip-assisted SERS-based chemical sensing and single biological cell studies. Anal Chim Acta 1317, 342903 (2024). doi: 10.1016/j.aca.2024.342903 [19] Wang XH, Hofmann O, Das R et al. Integrated thin-film polymer/fullerene photodetectors for on-chip microfluidic chemiluminescence detection. Lab Chip 7, 58–63 (2007). doi: 10.1039/B611067C [20] Fan MK, Wang PH, Escobedo C et al. Surface-enhanced Raman scattering (SERS) optrodes for multiplexed on-chip sensing of Nile blue A and oxazine 720. Lab Chip 12, 1554–1560 (2012). doi: 10.1039/c2lc20648j [21] Bai S, Ren XL, Obata K et al. Label-free trace detection of bio-molecules by liquid-interface assisted surface-enhanced Raman scattering using a microfluidic chip. Opto-Electron Adv 5, 210121 (2022). doi: 10.29026/oea.2022.210121 [22] Bo H, Ke Y, Yong Z et al. Microfluidic integrated D-shaped optical fiber SERS probe with high sensitivity and ability of multi-molecule detection. Opt Express 31 , 27304–27311 (2023). [23] Li L, Xiao L, Wang JH et al. Movable electrowetting optofluidic lens for optical axial scanning in microscopy. Opto-Electron Adv 2, 180025 (2019). [24] Sun N, Huang B, Lv ZY et al. UV-catalyzed TiO2-based optofluidic SERS chip for three online strategies: fabrication, detection, and self-cleaning. Anal Chem 96, 9104–9112 (2024). doi: 10.1021/acs.analchem.4c00657 [25] Huang JA, Zhang YL, Ding H et al. SERS-enabled lab-on-a-chip systems. Adv Opt Mater 3, 618–633 (2015). doi: 10.1002/adom.201400534 [26] Oliveira K, Teixeira A, Fernandes JM et al. Multiplex SERS phenotyping of single cancer cells in microdroplets. Adv Opt Mater 11, 2201500 (2023). doi: 10.1002/adom.202201500 [27] Na R, Xing W, Yuan G et al. Optofluidic SERS based on Ag nanocubes with high sensitivity for detecting a prevalent water pollutant. Opt Lett 49 , 2689–2692 (2024). [28] Deng YL, Juang YJ. Electrokinetic trapping and surface enhanced Raman scattering detection of biomolecules using optofluidic device integrated with a microneedles array. Biomicrofluidics 7, 014111 (2013). doi: 10.1063/1.4793224 [29] Chon H, Lim C, Ha SM et al. On-chip immunoassay using surface-enhanced Raman scattering of hollow gold nanospheres. Anal Chem 82, 5290–5295 (2010). doi: 10.1021/ac100736t [30] Zhao LY, Wang YL, Jin ST et al. Rational electrochemical design of hierarchical microarchitectures for SERS sensing applications. Nat Synth 3, 867–877 (2024). doi: 10.1038/s44160-024-00553-1 [31] Chen XY, Ding QQ, Bi C et al. Lossless enrichment of trace analytes in levitating droplets for multiphase and multiplex detection. Nat Commun 13, 7807 (2022). doi: 10.1038/s41467-022-35495-9 [32] Ashkin A. Acceleration and trapping of particles by radiation pressure. Phys Rev Lett 24, 156–159 (1970). doi: 10.1103/PhysRevLett.24.156 [33] Ashkin A. Optical trapping and manipulation of neutral particles using lasers. Proc Natl Acad Sci USA 94, 4853–4860 (1997). doi: 10.1073/pnas.94.10.4853 [34] Grier DG. A revolution in optical manipulation. Nature 424, 810–816 (2003). doi: 10.1038/nature01935 [35] Dholakia K, Reece P. Optical micromanipulation takes hold. Nano Today 1, 18–27 (2006). [36] Ashkin A. Forces of a single-beam gradient laser trap on a dielectric sphere in the ray optics regime. Biophys J 61, 569–582 (1992). doi: 10.1016/S0006-3495(92)81860-X [37] Bosanac L, Aabo T, Bendix PM et al. Efficient optical trapping and visualization of silver nanoparticles. Nano Lett 8, 1486–1491 (2008). doi: 10.1021/nl080490+ [38] Xin HB, Li BJ. Targeted delivery and controllable release of nanoparticles using a defect-decorated optical nanofiber. Opt Express 19, 13285–13290 (2011). doi: 10.1364/OE.19.013285 [39] Svedberg F, Li ZP, Xu HX et al. Creating hot nanoparticle pairs for surface-enhanced Raman spectroscopy through optical manipulation. Nano Lett 6, 2639–2641 (2006). doi: 10.1021/nl062101m [40] Tong LM, Righini M, Gonzalez MU et al. Optical aggregation of metal nanoparticles in a microfluidic channel for surface-enhanced Raman scattering analysis. Lab Chip 9, 193–195 (2009). doi: 10.1039/B813204F [41] Svedberg F, Käll M. On the importance of optical forces in surface-enhanced Raman scattering (SERS). Faraday Discuss 132, 35–44 (2006). doi: 10.1039/B509301P [42] Ashkin A, Dziedzic JM, Bjorkholm JE et al. Observation of a single-beam gradient force optical trap for dielectric particles. Opt Lett 11, 288–290 (1986). doi: 10.1364/OL.11.000288 [43] Xin HB, Li XM, Li BJ. Massive photothermal trapping and migration of particles by a tapered optical fiber. Opt Express 19, 17065–17074 (2011). doi: 10.1364/OE.19.017065 [44] Xin HB, Lei HX, Zhang Y et al. Photothermal trapping of dielectric particles by optical fiber-ring. Opt Express 19, 2711–2719 (2011). doi: 10.1364/OE.19.002711 [45] Harada Y, Asakura T. Radiation forces on a dielectric sphere in the Rayleigh scattering regime. Opt Commun 124, 529–541 (1996). doi: 10.1016/0030-4018(95)00753-9 -
Supplementary Information
Supplementary information for Single-beam optical trap-based surfaceenhanced raman scattering optofluidic molecular fingerprint spectroscopy detection system 
-
Access History
Article Metrics
-
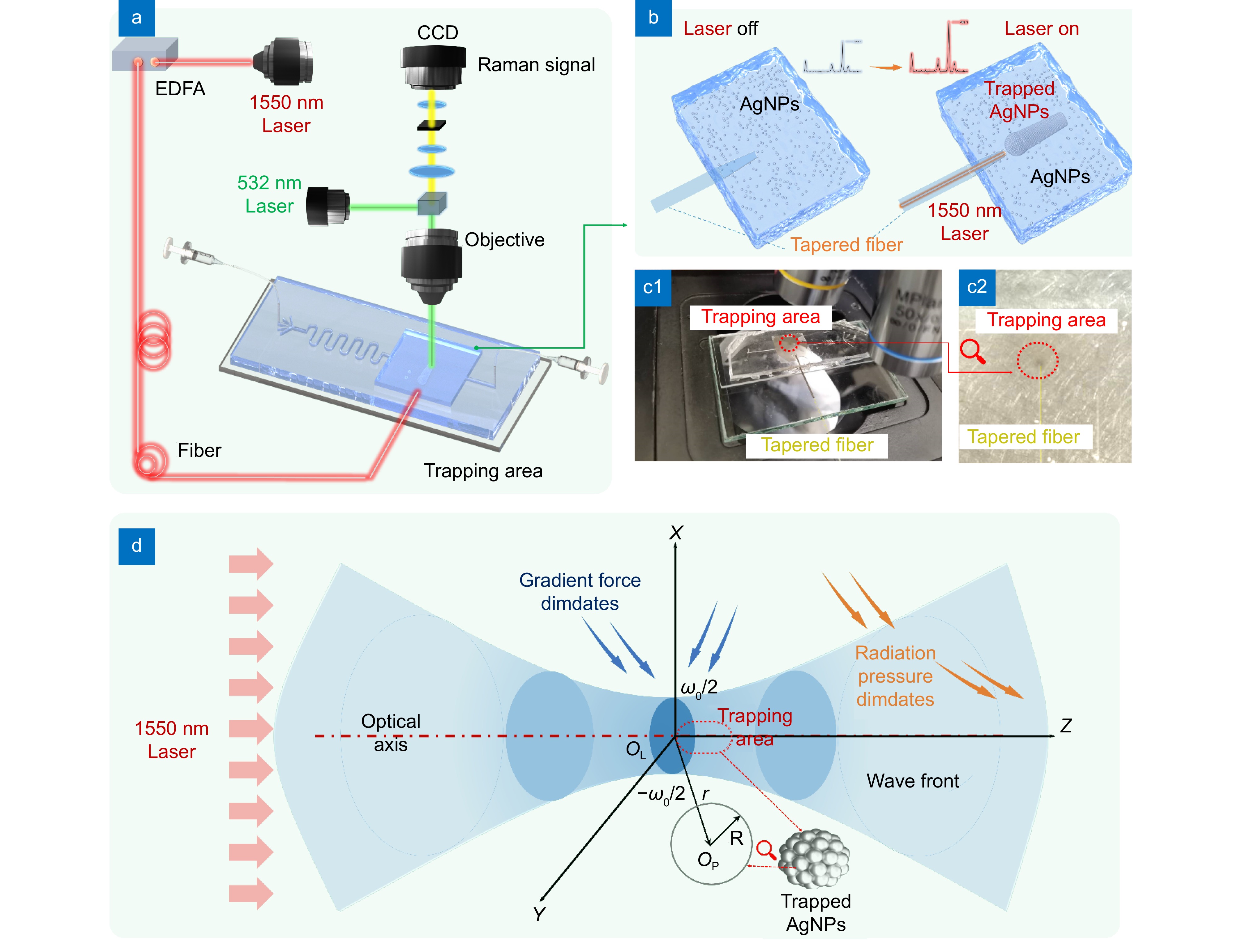
Figure 1.
(a) Schematic diagram of the optical trapping-enhanced SERS optofluidic detection system. (b) Effect of the single-beam optical trap module switch state on AgNPs aggregation. (c1) and (c2) Operational images of the single-beam optical trap-enhanced SERS optofluidic detection system, demonstrating particle trapping and Raman detection, with (c2) providing an enlarged view of the trapping area in (c1). (d) Schematic diagram of the optical trapping mechanism.
-
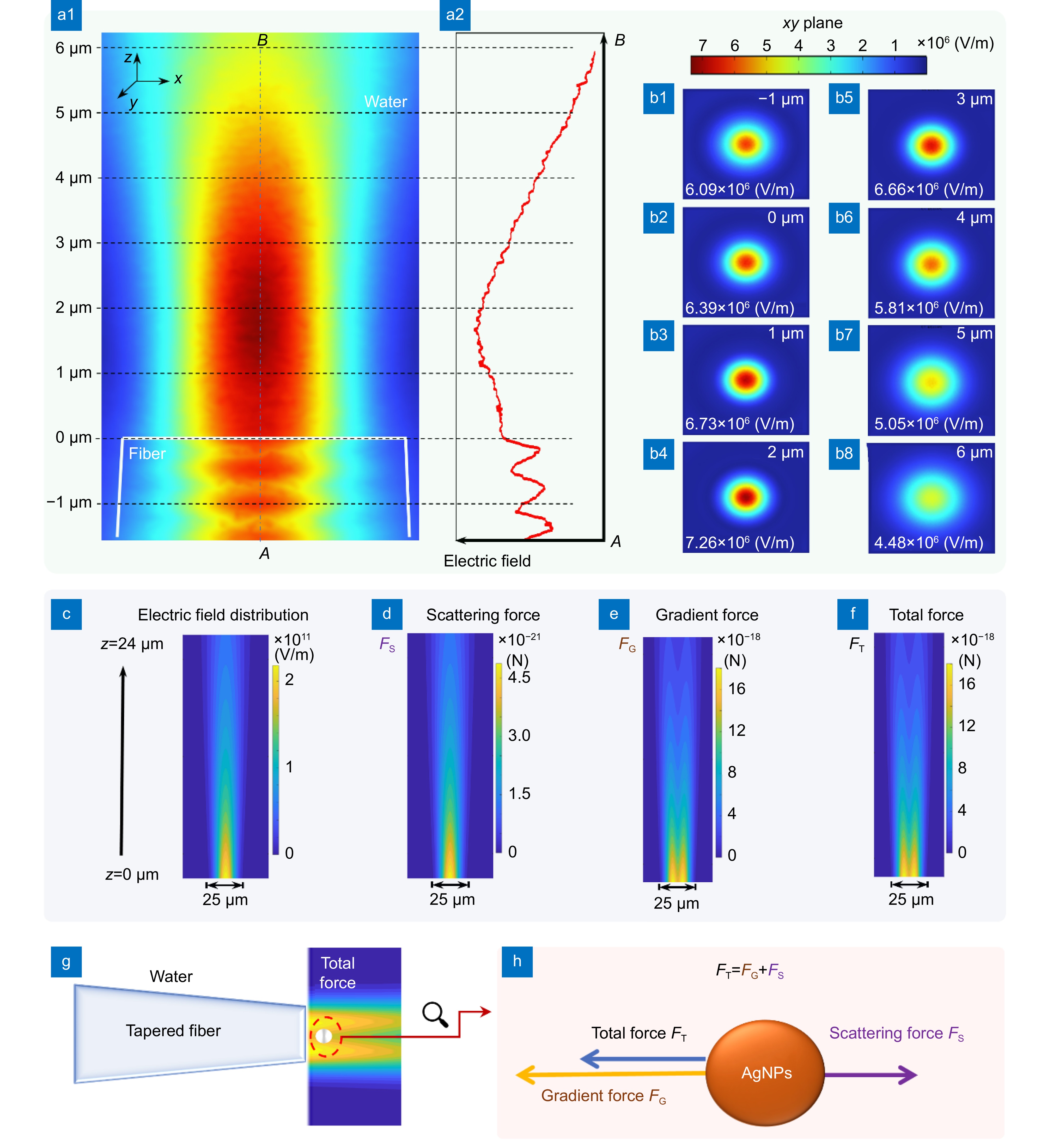
Figure 2.
(a1, a2) The simulation results of the variation in electric field intensity with distance for the 1550 nm laser emitted from the tapered optical fiber. (b1–b8) Respectively display the electric field intensity distribution at different positions. (c) Electric field distribution of 1550 nm laser emitted by a tapered fiber with a tip diameter of 25 μm in water. (d) Scattering force on 50 nm AgNPs. (e) Gradient force on 50 nm AgNPs. (f) Total force on 50 nm AgNPs. (g) AgNPs are strongly trapped near the tip of the tapered optical fiber. (h) Schematic diagram of the force analysis on AgNPs.
-
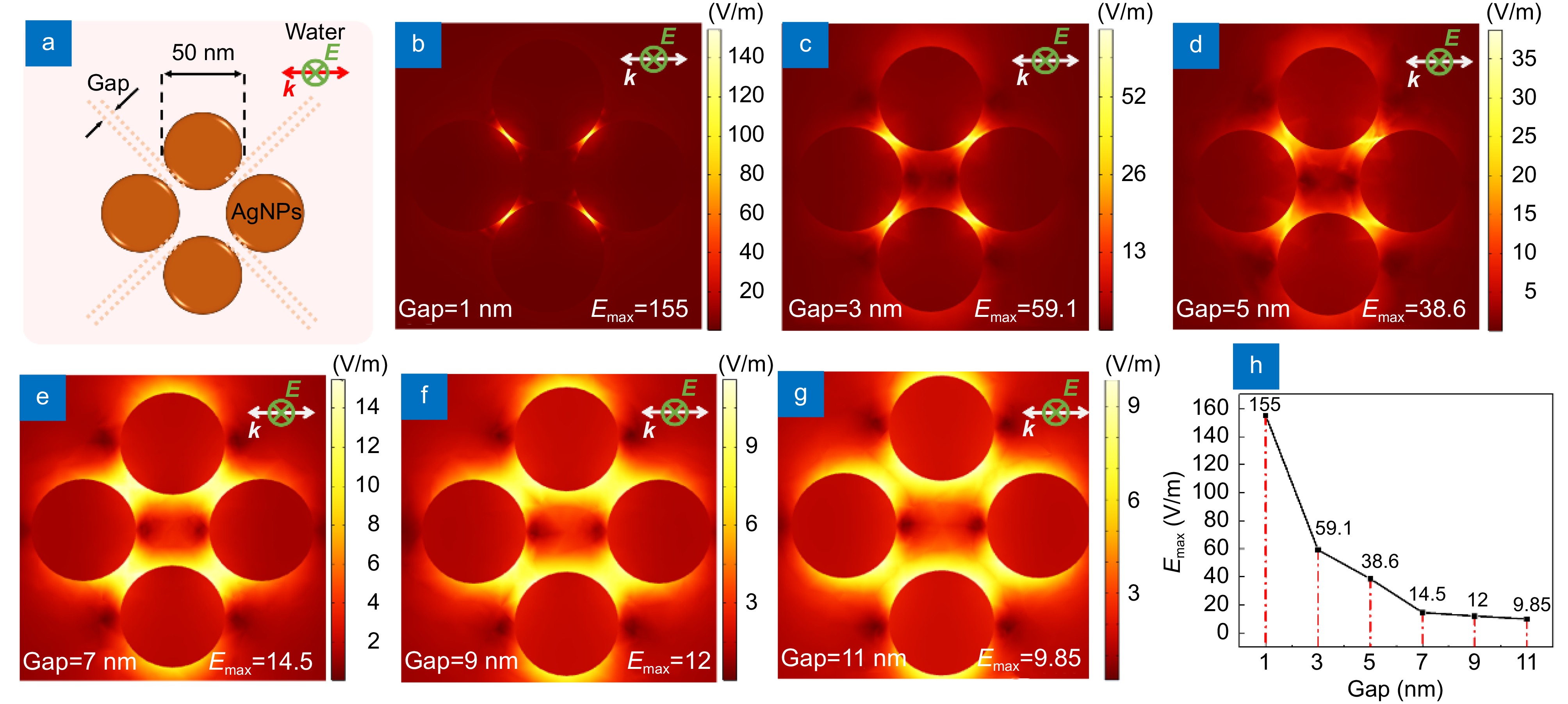
Figure 3.
(a) Electric field simulation model of 50 nm diameter AgNPs under 532 nm laser excitation with different gaps. (b) Electric field distribution when the gap is 1 nm. Electric field distribution when the gap is (c) 3 nm, (d) 5 nm, (e) 7 nm, (f) 9 nm and (g) 11 nm. (h) The maximal electric field as the gap between AgNPs decreased.
-
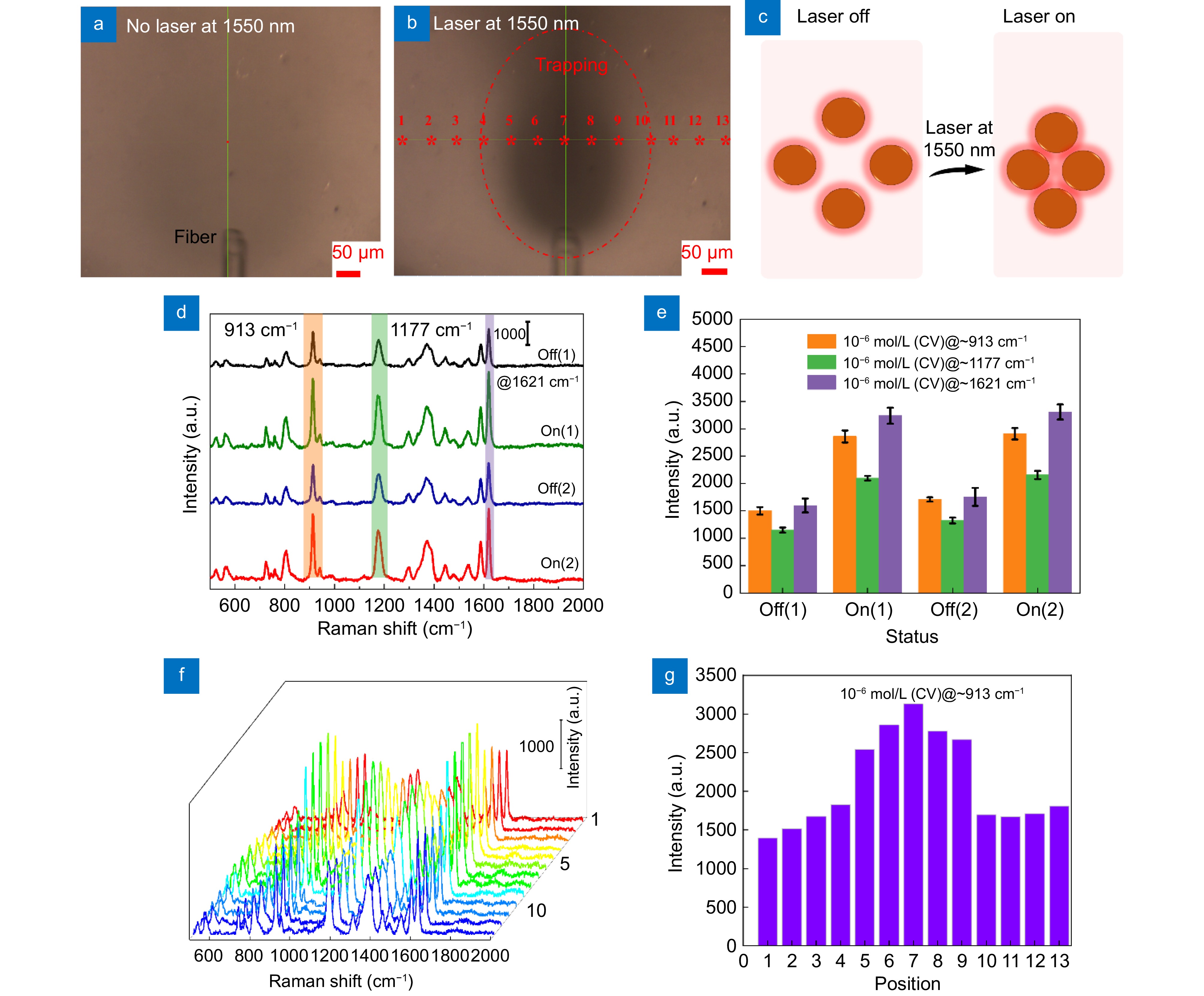
Figure 4.
Image observed by confocal Raman spectrometer microscope (a) when the single-beam optical trap is closed, with the detection region filled with a homogeneous and stable mixed solution. (b) When the single-beam optical trap is opened, with substances in the mixed solution captured by the optical trap emitted from the tapered fiber optical fiber. (c) Schematic diagram of AgNPs gap when the single-beam optical trap is off and on. (d) Raman spectra of 10−6 mol/L CV obtained by opening and closing the single-beam optical trap of position 7 in (b). (e) Raman characteristic peak intensity at 913, 1177 and 1621 cm−1 in (d). (f) Raman line mapping obtained at different positions when the optical trap is open. (g) Raman characteristic peak intensity at 913 cm−1 in (f).
-
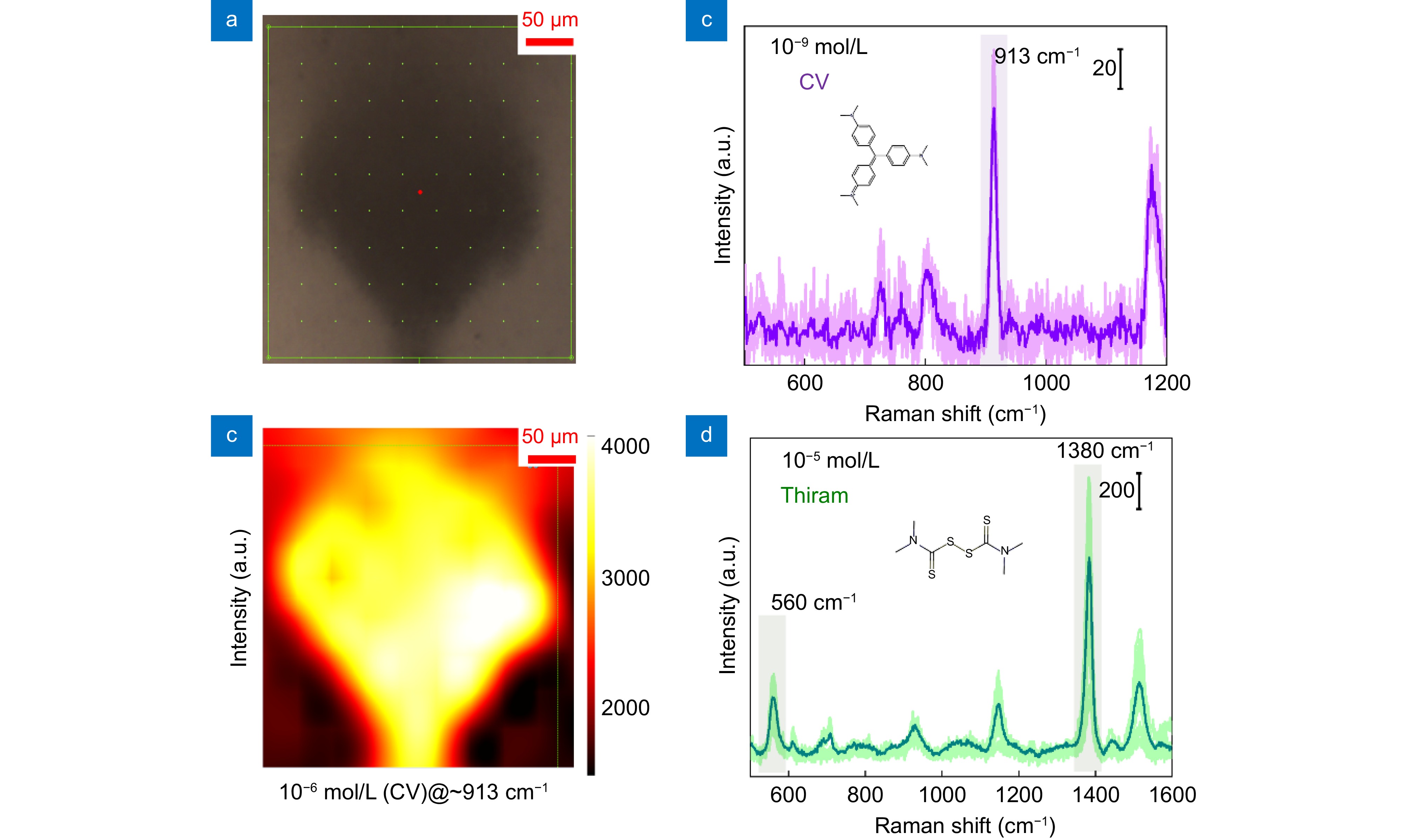
Figure 5.
(a) Positions of the Raman mapping. (b) Raman characteristic peak intensity at 913 cm−1 at different positions in (a). (c) Detection limit of the system for CV at a concentration of 10−9 mol/L. (d) Detection limit of the system for thiram at a concentration of 10−5 mol/L.

 E-mail Alert
E-mail Alert RSS
RSS

 DownLoad:
DownLoad: