| Citation: | Xu ZQ, Kang YK, Zhang J et al. NIR-triggered on-site NO/ROS/RNS nanoreactor: Cascade-amplified photodynamic/photothermal therapy with local and systemic immune responses activation. Opto-Electron Adv 7, 240013 (2024). doi: 10.29026/oea.2024.240013 |
NIR-triggered on-site NO/ROS/RNS nanoreactor: Cascade-amplified photodynamic/photothermal therapy with local and systemic immune responses activation
-
Abstract
Photothermal and photodynamic therapies (PTT/PDT) hold promise for localized tumor treatment, yet their full potential is hampered by limitations such as the hypoxic tumor microenvironment and inadequate systemic immune activation. Addressing these challenges, we present a novel near-infrared (NIR)-triggered RNS nanoreactor (PBNO-Ce6) to amplify the photodynamic and photothermal therapy efficacy against triple-negative breast cancer (TNBC). The designed PBNO-Ce6 combines sodium nitroprusside-doped Prussian Blue nanoparticles with Chlorin e6 to enable on-site RNS production through NIR-induced concurrent NO release and ROS generation. This not only enhances tumor cell eradication but also potentiates local and systemic antitumor immune responses, protecting mice from tumor rechallenge. Our in vivo evaluations revealed that treatment with PBNO-Ce6 leads to a remarkable 2.7-fold increase in cytotoxic T lymphocytes and a 62% decrease in regulatory T cells in comparison to the control PB-Ce6 (Prussian Blue nanoparticles loaded with Chlorin e6), marking a substantial improvement over traditional PTT/PDT. As such, the PBNO-Ce6 nanoreactor represents a transformative approach for improving outcomes in TNBC and potentially other malignancies affected by similar barriers. -
-
References
[1] Overchuk M, Weersink RA, Wilson BC et al. Photodynamic and photothermal therapies: synergy opportunities for nanomedicine. ACS Nano 17, 7979–8003 (2023). doi: 10.1021/acsnano.3c00891 [2] Shen S, Qiu JC, Huo D et al. Nanomaterial-enabled photothermal heating and its use for cancer therapy via localized hyperthermia. Small 20, 2305426 (2024). doi: 10.1002/smll.202305426 [3] Yang HY, Liu RF, Xu YX et al. Photosensitizer nanoparticles boost photodynamic therapy for pancreatic cancer treatment. Nanomicro Lett 13, 35 (2021). [4] Hu TT, Wang ZD, Shen WC et al. Recent advances in innovative strategies for enhanced cancer photodynamic therapy. Theranostics 11, 3278–3300 (2021). doi: 10.7150/thno.54227 [5] Lee D, Kwon S, Jang SY et al. Overcoming the obstacles of current photodynamic therapy in tumors using nanoparticles. Bioact Mater 8, 20–34 (2022). [6] Yuan C, Li Y, Xu ZQ et al. Imaging-guided synergistic photo-chemotherapy using doxorubicin-loaded gadolinium porphyrin-based metal-organic framework nanosheets. ACS Appl Nano Mater 5, 15318–15327 (2022). doi: 10.1021/acsanm.2c03390 [7] Wang SJ, Huang P, Nie LM et al. Single continuous wave laser induced photodynamic/plasmonic photothermal therapy using photosensitizer-functionalized gold nanostars. Adv Mater 25, 3055–3061 (2013). doi: 10.1002/adma.201204623 [8] Yang S, Sun B, Liu F et al. NIR-II imaging-guided mitochondrial-targeting organic nanoparticles for multimodal synergistic tumor therapy. Small 19, 2207995 (2023). doi: 10.1002/smll.202207995 [9] Yue J, Mei Q, Wang PY et al. Light-triggered multifunctional nanoplatform for efficient cancer photo-immunotherapy. J Nanobiotechnol 20, 181 (2022). doi: 10.1186/s12951-022-01388-8 [10] Li ZL, Lai XQ, Fu SQ et al. Immunogenic cell death activates the tumor immune microenvironment to boost the immunotherapy efficiency. Adv Sci (Weinh) 9, 2201734 (2022). doi: 10.1002/advs.202201734 [11] Wang MY, He MY, Zhang MY et al. Controllable hypoxia-activated chemotherapy as a dual enhancer for synergistic cancer photodynamic immunotherapy. Biomaterials 301, 122257 (2023). doi: 10.1016/j.biomaterials.2023.122257 [12] Zhao M, Zhang YY, Miao J et al. An activatable phototheranostic probe for anti-hypoxic type i photodynamic- and immuno-therapy of cancer. Adv Mater 36, 2305243 (2024). doi: 10.1002/adma.202305243 [13] Yun HY, Dawson VL, Dawson TM. Nitric oxide in health and disease of the nervous system. Mol Psychiatry 2, 300–310 (1997). doi: 10.1038/sj.mp.4000272 [14] Marletta MA. Nitric oxide: biosynthesis and biological significance. Trends Biochem Sci 14, 488–492 (1989). doi: 10.1016/0968-0004(89)90181-3 [15] Chang MY, Wang M, Liu YH et al. Dendritic plasmonic CuPt alloys for closed-loop multimode cancer therapy with remarkably enhanced efficacy. Small 19, 2206423 (2023). doi: 10.1002/smll.202206423 [16] Chen Y, Li ZH, Pan P et al. Tumor-specific ONOO– nanogenerator for improved drug delivery and enhanced chemotherapy of tumor. ACS Nano 15, 11514–11525 (2021). doi: 10.1021/acsnano.1c01312 [17] Shi MH, Zhang JL, Wang Y et al. Tumor-specific nitric oxide generator to amplify peroxynitrite based on highly penetrable nanoparticles for metastasis inhibition and enhanced cancer therapy. Biomaterials 283, 121448 (2022). doi: 10.1016/j.biomaterials.2022.121448 [18] Ji CW, Si JX, Xu Y et al. Mitochondria-targeted and ultrasound-responsive nanoparticles for oxygen and nitric oxide codelivery to reverse immunosuppression and enhance sonodynamic therapy for immune activation. Theranostics 11, 8587–8604 (2021). doi: 10.7150/thno.62572 [19] Beurton J, Boudier A, Barozzi Seabra A et al. Nitric oxide delivering surfaces: an overview of functionalization strategies and efficiency progress. Adv Healthc Mater 11, 2102692 (2022). doi: 10.1002/adhm.202102692 [20] van der Vlies AJ, Yamane S, Hasegawa U. Recent advance in self-assembled polymeric nanomedicines for gaseous signaling molecule delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol 16, e1934 (2024). doi: 10.1002/wnan.1934 [21] Gao D, Asghar S, Hu RF et al. Recent advances in diverse nanosystems for nitric oxide delivery in cancer therapy. Acta Pharm Sin B 13, 1498–1521 (2023). doi: 10.1016/j.apsb.2022.11.016 [22] Tavakkoli Yaraki M, Liu B, Tan YN. Emerging strategies in enhancing singlet oxygen generation of nano-photosensitizers toward advanced phototherapy. Nanomicro Lett 14, 123 (2022). [23] So JY, Ohm J, Lipkowitz S et al. Triple negative breast cancer (TNBC): non-genetic tumor heterogeneity and immune microenvironment: emerging treatment options. Pharmacol Ther 237, 108253 (2022). doi: 10.1016/j.pharmthera.2022.108253 [24] Jia HY, Truica CI, Wang B et al. Immunotherapy for triple-negative breast cancer: existing challenges and exciting prospects. Drug Resist Updat 32, 1–15 (2017). doi: 10.1016/j.drup.2017.07.002 [25] Chen X, Iliopoulos D, Zhang Q et al. XBP1 promotes triple-negative breast cancer by controlling the HIF1α pathway. Nature 508, 103–107 (2014). doi: 10.1038/nature13119 [26] Ding MC, Shao K, Wu LJ et al. A NO/ROS/RNS cascaded-releasing nano-platform for gas/PDT/PTT/immunotherapy of tumors. Biomater Sci 9, 5824–5840 (2021). doi: 10.1039/D1BM00726B [27] Ma SJ, Zhao Y, Lee WC et al. Hypoxia induces HIF1α-dependent epigenetic vulnerability in triple negative breast cancer to confer immune effector dysfunction and resistance to anti-PD-1 immunotherapy. Nat Commun 13, 4118 (2022). doi: 10.1038/s41467-022-31764-9 [28] Thomas AA, Fisher JL, Rahme GJ et al. Regulatory T cells are not a strong predictor of survival for patients with glioblastoma. Neuro Oncol 17, 801–809 (2015). doi: 10.1093/neuonc/nou363 [29] Zhang J, Xu ZQ, Li Y et al. Theranostic mesoporous platinum nanoplatform delivers halofuginone to remodel extracellular matrix of breast cancer without systematic toxicity. Bioeng Transl Med 8, e10427 (2023). doi: 10.1002/btm2.10427 [30] Gotwals P, Cameron S, Cipolletta D et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat Rev Cancer 17, 286–301 (2017). doi: 10.1038/nrc.2017.17 [31] Racle J, de Jonge K, Baumgaertner P et al. Simultaneous enumeration of cancer and immune cell types from bulk tumor gene expression data. eLife 6, e26476 (2017). doi: 10.7554/eLife.26476 [32] Feng T, Wan JY, Li P et al. A novel NIR-controlled NO release of sodium nitroprusside-doped Prussian blue nanoparticle for synergistic tumor treatment. Biomaterials 214, 119213 (2019). doi: 10.1016/j.biomaterials.2019.05.024 [33] Wang SJ, Ma XQ, Hong XH et al. Adjuvant photothermal therapy inhibits local recurrences after breast-conserving surgery with little skin damage. ACS Nano 12, 662–670 (2018). doi: 10.1021/acsnano.7b07757 [34] Tallarida RJ. Quantitative methods for assessing drug synergism. Genes Cancer 2, 1003–1008 (2011). doi: 10.1177/1947601912440575 [35] Wu QH, You L, Nepovimova E et al. Hypoxia-inducible factors: master regulators of hypoxic tumor immune escape. J Hematol Oncol 15, 77 (2022). doi: 10.1186/s13045-022-01292-6 [36] Wei HM, Xu ZM, Chen LC et al. Long non-coding RNA PAARH promotes hepatocellular carcinoma progression and angiogenesis via upregulating HOTTIP and activating HIF-1α/VEGF signaling. Cell Death Dis 13, 102 (2022). doi: 10.1038/s41419-022-04505-5 [37] Hoskin PJ, Sibtain A, Daley FM et al. GLUT1 and CAIX as intrinsic markers of hypoxia in bladder cancer: relationship with vascularity and proliferation as predictors of outcome of ARCON. Br J Cancer 89, 1290–1297 (2003). doi: 10.1038/sj.bjc.6601260 [38] Lynch MS, Cheng M, Van Kuiken BE et al. Probing the photoinduced metal-nitrosyl linkage isomerism of sodium nitroprusside in solution using transient infrared spectroscopy. J Am Chem Soc 133, 5255–5262 (2011). doi: 10.1021/ja110881n -
Supplementary Information
Supplementary information for NIR-triggered on-site NO/ROS/RNS nanoreactor: Cascade-amplified photodynamic/photothermal therapy with local and systemic immune responses activation 
-
Access History
Article Metrics
-
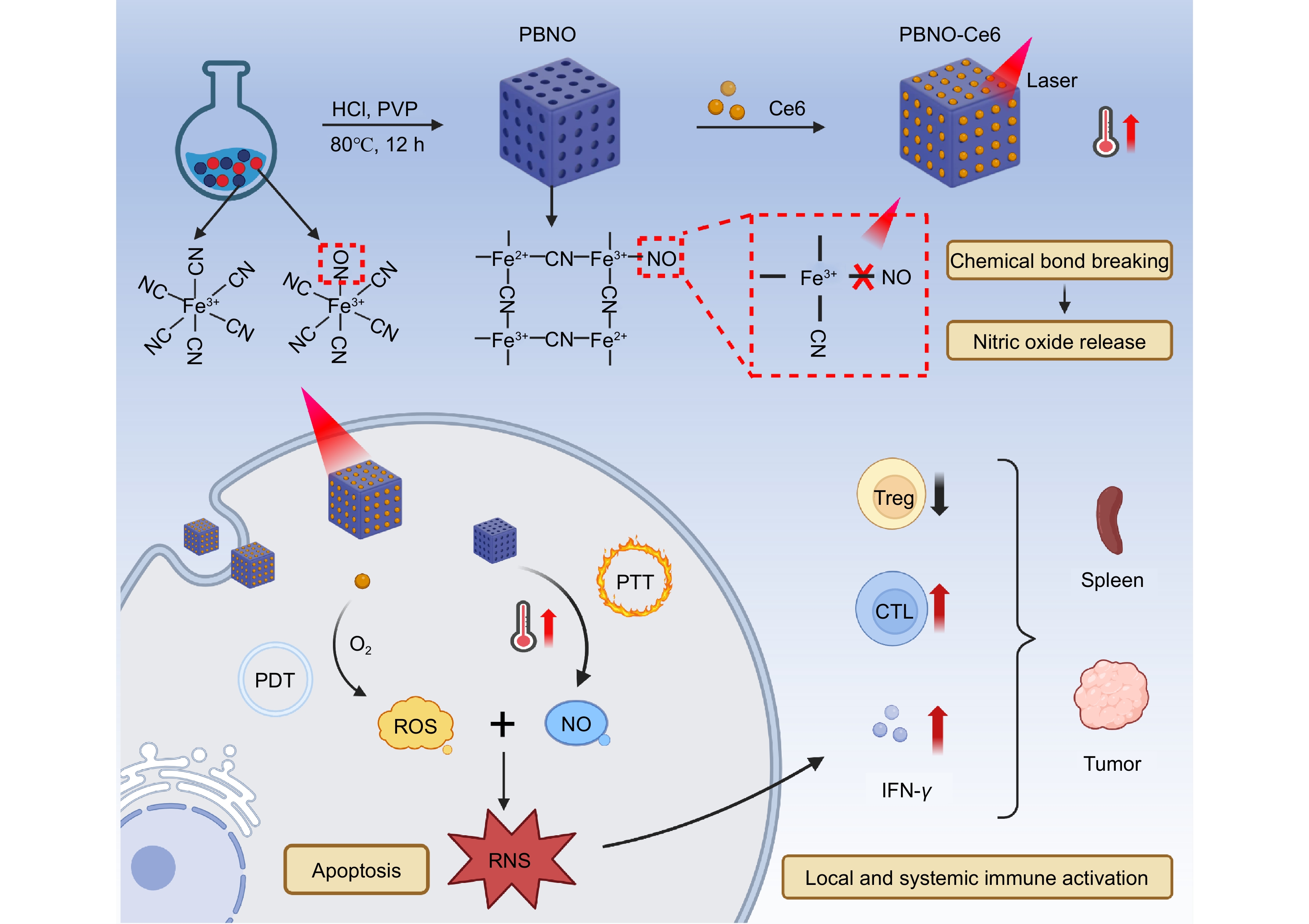
Figure 1.
Illustration showing the preparation and use of the RNS nanoreactor. The PBNO-Ce6 nanoparticles heat up (PTT) and release NO when exposed to NIR laser. At the same time, ROS are generated (PDT) and further transform the NO into RNS. The combined effect of PTT and RNS-enhanced PDT causes cell apoptosis and promotes strong activation of local and systemic immune responses.
-
Figure 1.
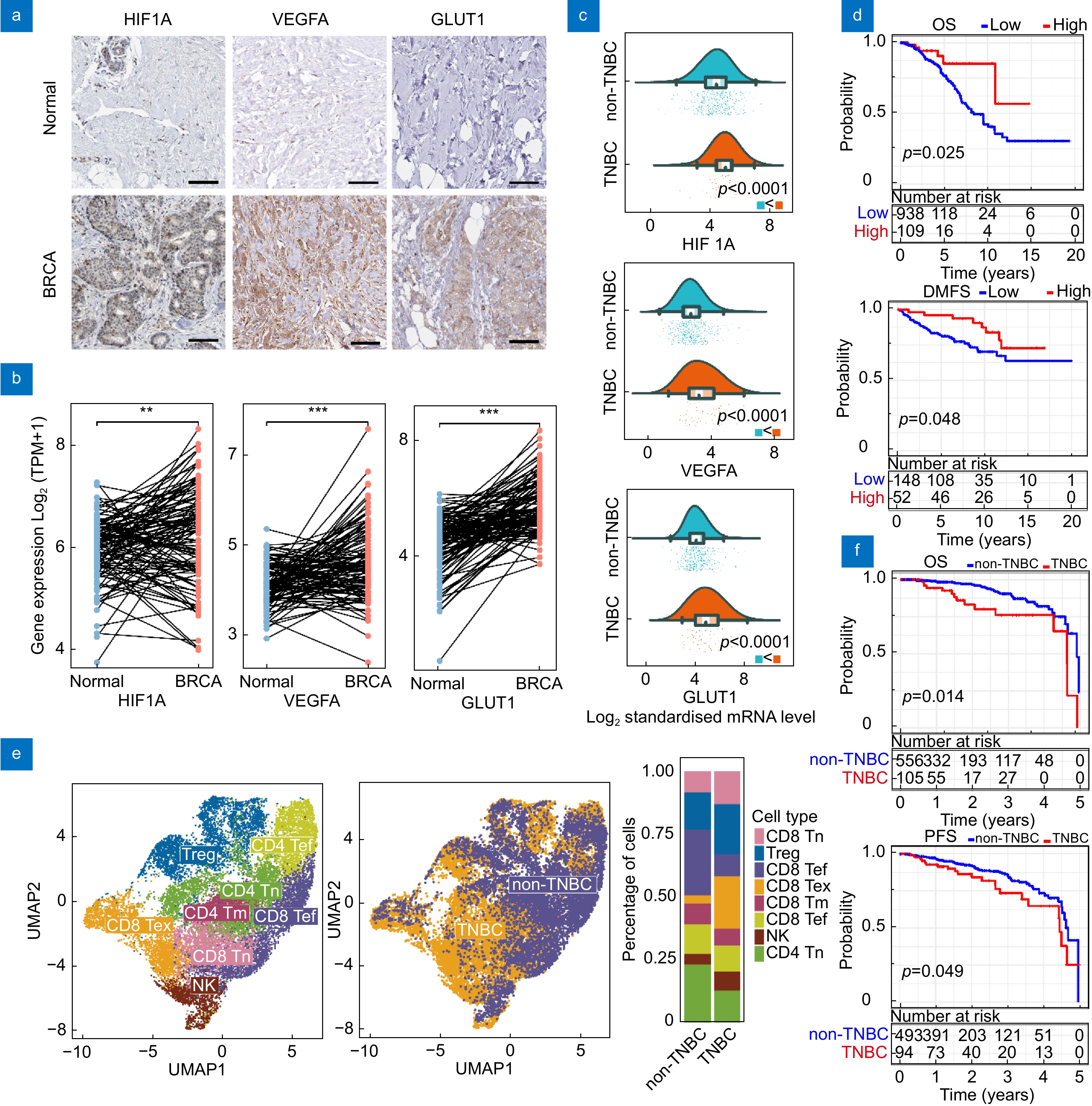
Bioinformatics analysis of breast cancer patients. (a) Immunohistochemistry images showing HIF1A, VEGFA, and GLUT1 staining in breast cancer tumor tissue and normal breast tissue. Scale bar: 100 μm. (b) HIF1A, VEGFA, and GLUT1 gene expression profiles across TCGA/GTEx breast cancer datasets. (c) Differential expression of HIF1A, VEGFA, and GLUT1 in TNBC compared to non-TNBC. (d) Kaplan-Meier survival curves for OS and DMFS in patients with high vs low immune scores. (e) Single cell analysis of T cells in TNBC and non-TNBC tumors. (Tn: naive T cells; Tef: effector T cells; Tex: exhausted T cells; Tm: memory T cells; Tef: effector memory T Cell.) (f) Kaplan-Meier survival curves for OS and PFS in TNBC and non-TNBC patients.
-
Figure 2.
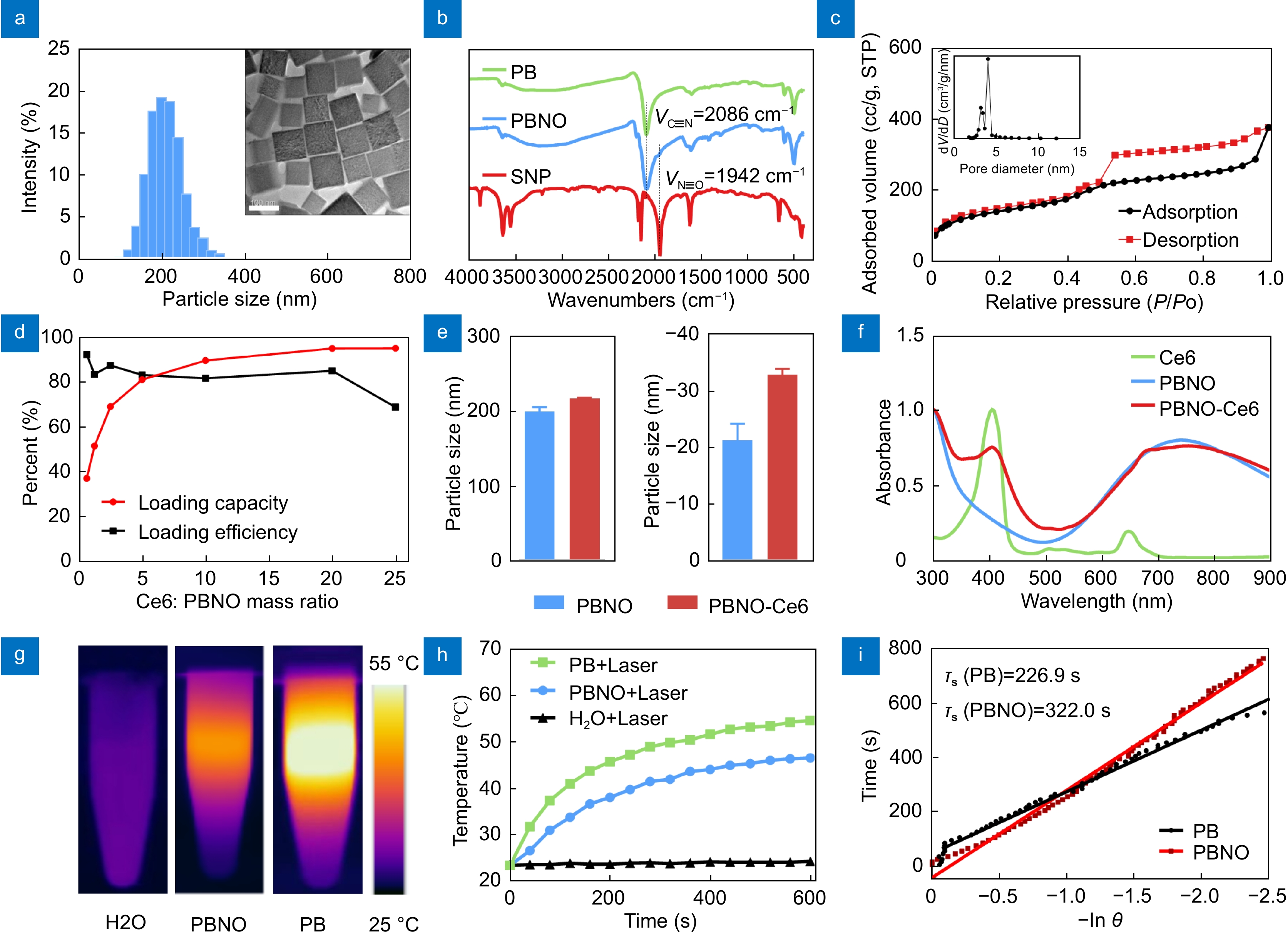
Characterization of PBNO-Ce6 nanoparticles. (a) Particle size distribution and TEM image of PBNO. (b) FT-IR spectra of PB, PBNO and SNP. (c) N2 adsorption-desorption isotherms of PBNO. (d) Loading capacity and efficiency of Ce6 on PBNO. (e) Size and zeta potential of PBNO and PBNO-Ce6. (f) UV-vis absorption spectra of PBNO, Ce6 and PBNO-Ce6. (g) Thermographs of H2O, PB and PBNO irradiated by 660-nm laser under 0.4 W cm−2. (h) Corresponding photothermal heating curves of H2O, PB and PBNO. (i) Linear time vs -lnθ plots obtained from the cooling period of PB and PBNO.
-
Figure 3.
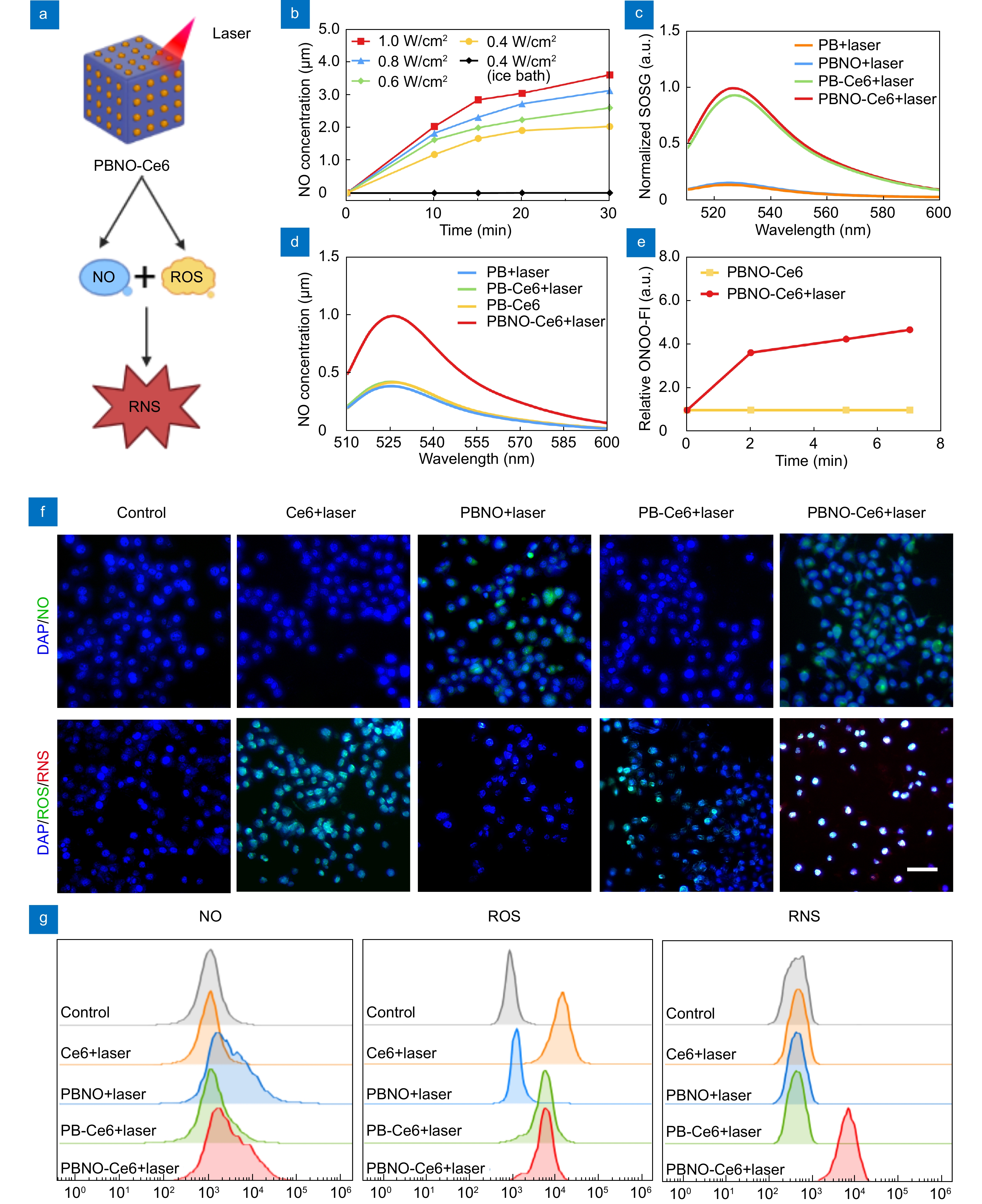
NIR triggered generation of NO/ROS/RNS by PBNO-Ce6. (a) Scheme showing NO/ROS/RNS generation. (b) NO release detected after laser irradiation of PBNO. (c) ROS generation detected by SOSG fluorescence of PB, PBNO, PB-Ce6, and PBNO-Ce6 after laser irradiation. (d) RNS generation detected by BBoxiProbe® O56 fluorescence of PB, PBNO, PB-Ce6, and PBNO-Ce6 after laser irradiation. (e) BBoxiProbe® O56 fluorescence intensity over time. (f) Fluorescence images of 4T1 cells stained with probes for NO (DAM-FM DA), ROS (DCFH-DA) and RNS (BBoxiProbe® O52). Scale bar: 50 μm. (g) Corresponding flow cytometry analysis of 4T1 cells stained with DAM-FM DA, DCFH-DA and BBoxiProbe® O52.
-
Figure 4.
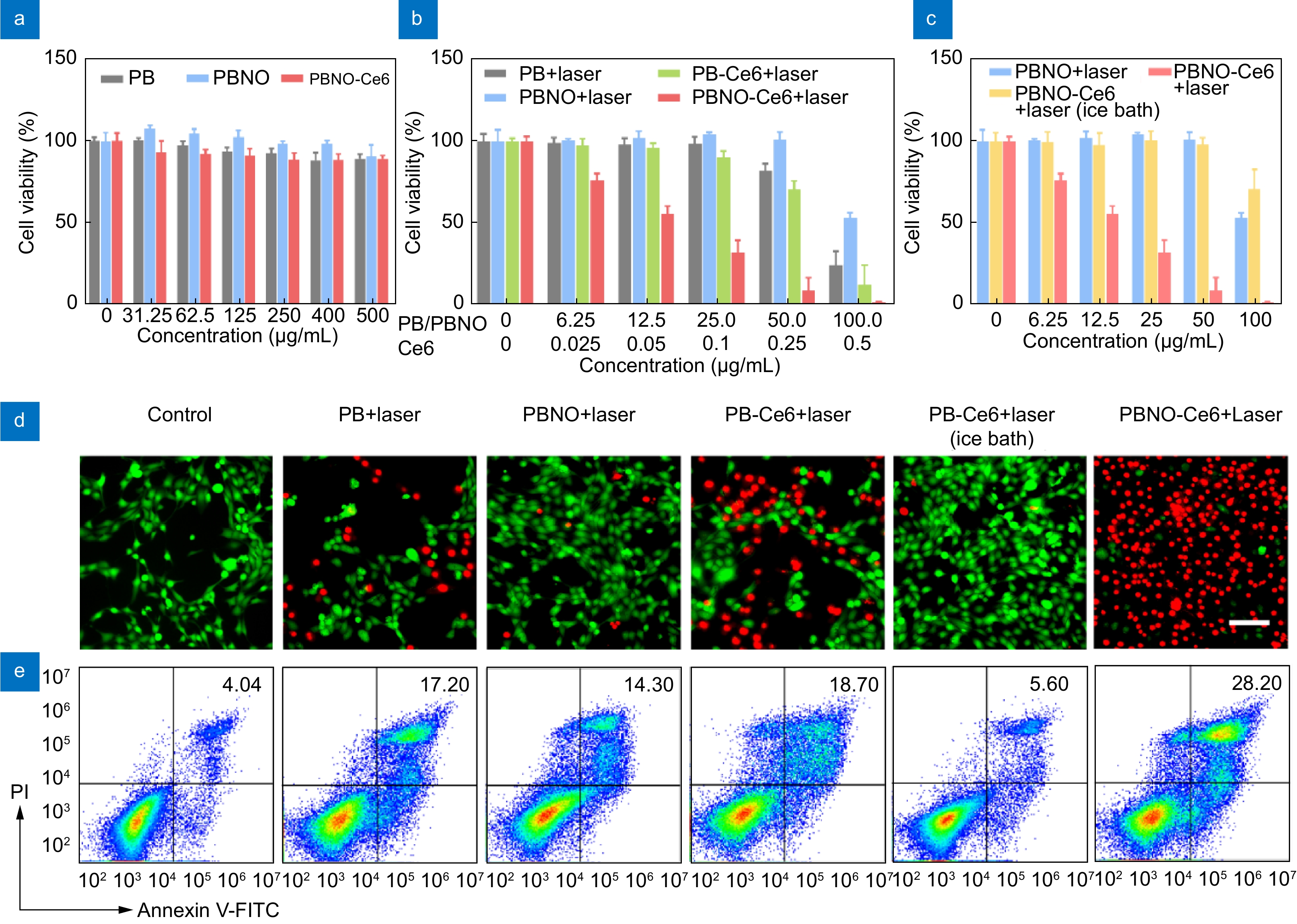
In vitro therapeutic efficacy of PBNO-Ce6. (a) Viability of 4T1 cells after 24 h incubation with PB, PBNO and PBNO-Ce6 without laser irradiation. (b) Viability of 4T1 cells after 24 h incubation with PB, PBNO, PB-Ce6 or PBNO-Ce6 and irradiated at 660 nm (0.4 W·cm−2, 5 min). (c) Viability of 4T1 cells incubated with PBNO and PBNO-Ce6 (with or without ice bath cooling) for 24 h and irradiated at 660 nm. (d) Fluorescence imaging of Calcein-AM and PI stained 4T1 cells after different treatments (concentration: 50 μg·mL−1). Green: Calcein-AM; Red: PI. Scale bar: 50 μm. (e) Flow cytometry analysis of apoptosis in 4T1 cells after different treatments (concentration: 25 μg·mL−1).
-
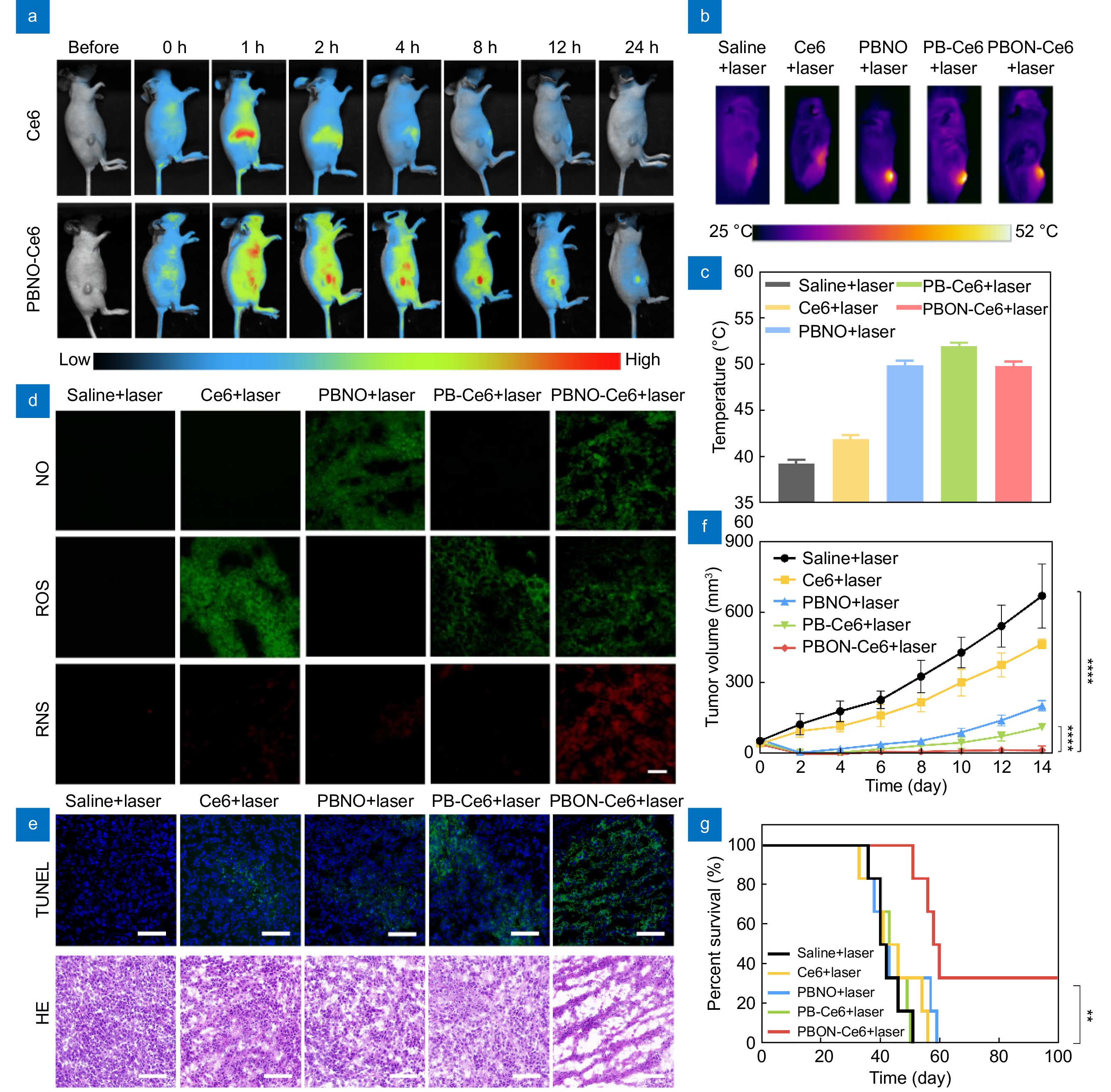
Figure 5.
In vivo therapeutic efficacy of PBNO-Ce6. (a) Fluorescence images of mice at different time points after intravenous injection of free Ce6 or PBNO-Ce6. (b) Thermal images of mice during laser irradiation. (c) Temperature changes at the tumor area after irradiation. (d) Fluorescence imaging of NO, ROS and RNS generation in tumors. (e) TUNEL and H&E staining of tumors after treatment. Scale bar: 50 μm. (f) Tumor growth curves of mice after different treatments. (g) Kaplan-Meier survival curves of mice in different treatment groups. (*p< 0.05, **p< 0.01,***p< 0.001, ****p< 0.0001)
-
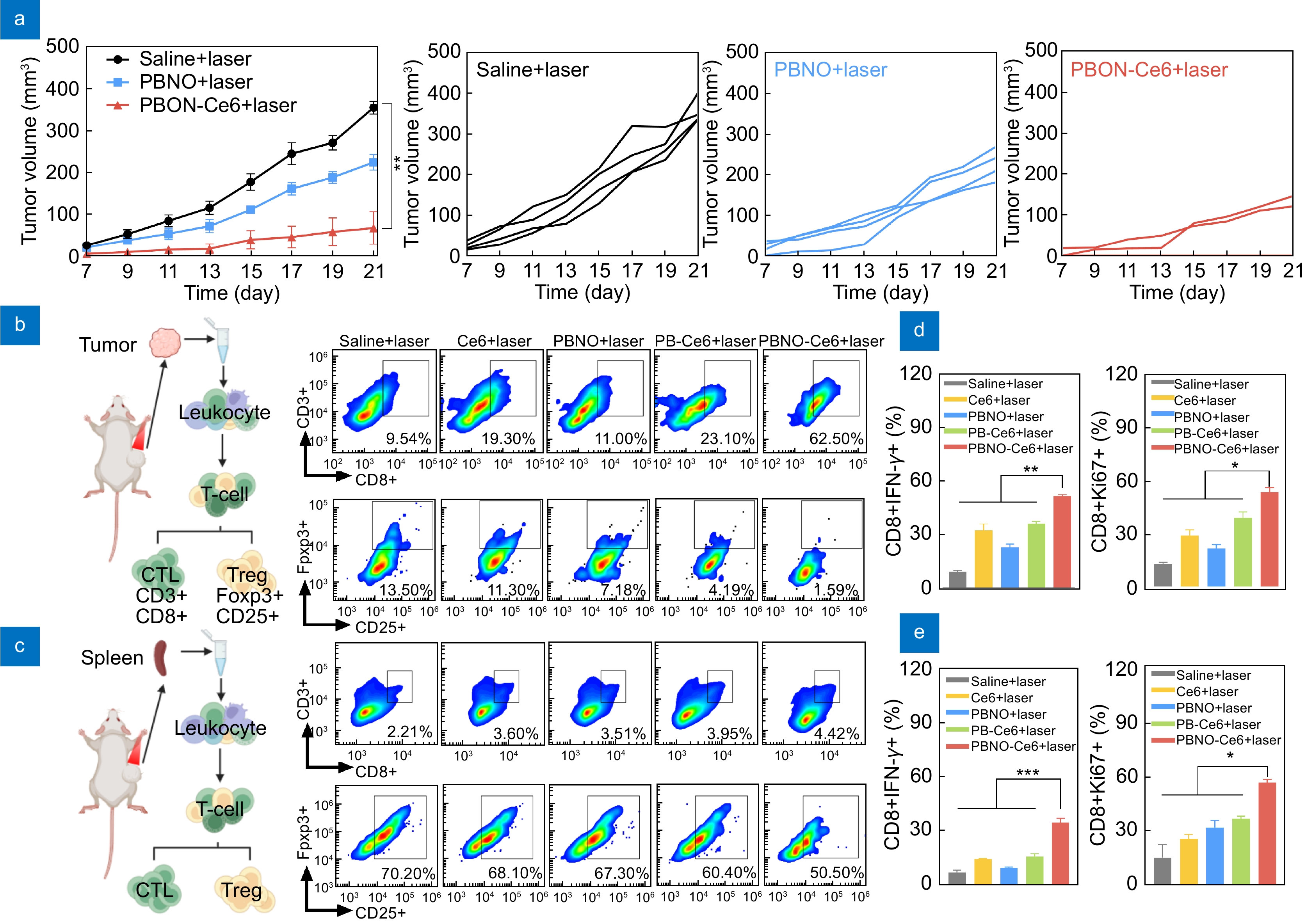
Figure 6.
Local and systemic antitumor immune responses induced by PBNO-Ce6 treatment. (a) Tumor growth curves of mice after tumors rechallenge in different treatment groups. (b, c) Flow cytometry analysis of immunosuppressive Tregs (CD4+Foxp3+CD25+) and antitumor CTLs (CD3+CD8+) in (b) primary tumors and (c) spleens. (d, e) Analysis of IFN-γ+ and Ki67+ expression in CTLs in (d) primary tumors and (e) spleens. (*p< 0.05, **p< 0.01,***p< 0.001, ****p< 0.0001)
-
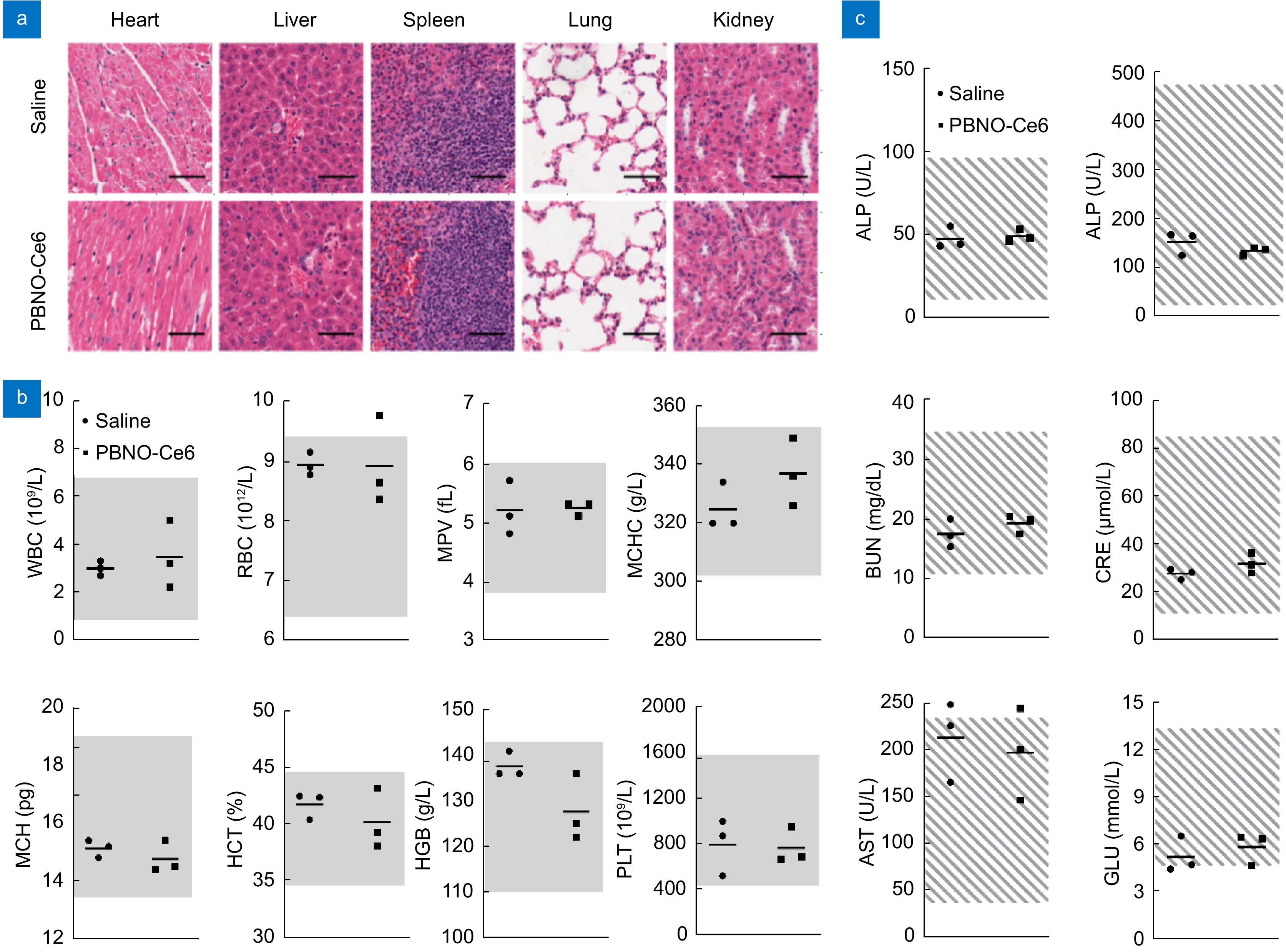
Figure 7.
Biocompatibility of PBNO-Ce6 in vivo. (a) H&E images of heart, liver, spleen, lung, and kidney tissues from mice at 14 days post-injection. Scale bar: 50 μm. (b) Routine blood cell count of mice at 14 days post-injection. WBC: white blood cells; RBC: red blood cells; MPV: mean platelet volume; MCHC: mean corpuscular hemoglobin concentration; MCH: mean corpuscular hemoglobin; HCT: hematocrit; HGB: hemoglobin; PLT: platelet count. The gray areas indicate the normal range of the indicators. (c) Blood biochemistry analysis of mice at 14 days post-injection. ALT: alanine aminotransferase; ALP: alkaline phosphatase; BUN: blood urea nitrogen; CRE: creatinine; AST: aspartate aminotransferase; GLU: glucose.

 E-mail Alert
E-mail Alert RSS
RSS

 DownLoad:
DownLoad: