| Citation: | Li JP, Liu XH, Xiao ZH et al. Broadband ultrasound generator over fiber-optic tip for in vivo emotional stress modulation. Opto-Electron Sci x, 240034 (2025). doi: 10.29026/oes.2025.240034 |
Broadband ultrasound generator over fiber-optic tip for in vivo emotional stress modulation
-
Abstract
Ultrasonic neuromodulation has gained recognition as a promising therapeutic approach. A miniature transducer capable of generating suitable-strength and broadband ultrasound is of great significance for achieving high spatial precision ultrasonic neural stimulation. However, the ultrasound transducer with the above integrated is yet to be challenged. Here, we developed a fiber-optic photoacoustic emitter (FPE) with a diameter of 200 μm, featuring controllable sound intensity and a broadband response (−6 dB bandwidth: 162%). The device integrates MXene (Ti3C2Tx), known for its exceptional photothermal properties, and polydimethylsiloxane, which offers a high thermal expansion coefficient. This FPE, exhibiting high spatial precision (lateral: 163.3 μm, axial: 207 μm), is capable of selectively activating neurons in targeted regions. Using the TetTagging method to selectively express a cfos-promoter-inducible mCHERRY gene within the medial prefrontal cortex (mPFC), we found that photoacoustic stimulation significantly and temporarily activated the neurons. In vivo fiber photometry demonstrated that photoacoustic stimulation induced substantial calcium transients in mPFC neurons. Furthermore, we confirmed that photoacoustic stimulation of the mPFC using FPE markedly alleviates acute social defeat stress-induced emotional stress in mice. This work demonstrates the potential of FPEs for clinical applications, with a particular focus on modulating neural activity to regulate emotions. -
-
References
[1] Hou XD, Jing JN, Jiang YZ et al. Nanobubble-actuated ultrasound neuromodulation for selectively shaping behavior in mice. Nat Commun 15, 2253 (2024). doi: 10.1038/s41467-024-46461-y [2] Piech DK, Johnson BC, Shen K et al. A wireless millimetre-scale implantable neural stimulator with ultrasonically powered bidirectional communication. Nat Biomed Eng 4, 207–222 (2020). doi: 10.1038/s41551-020-0518-9 [3] Shi LL, Jiang Y, Fernandez FR et al. Non-genetic photoacoustic stimulation of single neurons by a tapered fiber optoacoustic emitter. Light Sci Appl 10, 143 (2021). doi: 10.1038/s41377-021-00580-z [4] Cotero V, Graf J, Miwa H et al. Stimulation of the hepatoportal nerve plexus with focused ultrasound restores glucose homoeostasis in diabetic mice, rats and swine. Nat Biomed Eng 6, 683–705 (2022). doi: 10.1038/s41551-022-00870-w [5] Leinenga G, Langton C, Nisbet R et al. Ultrasound treatment of neurological diseases-current and emerging applications. Nat Rev Neurol 12, 161–174 (2016). doi: 10.1038/nrneurol.2016.13 [6] Yoon CW, Lee NS, Koo KM et al. Investigation of ultrasound-mediated intracellular Ca2+ oscillations in HIT-T15 pancreatic β-cell Line. Cells 9, 1129 (2020). doi: 10.3390/cells9051129 [7] Hou JF, Nayeem MOG, Caplan KA et al. An implantable piezoelectric ultrasound stimulator (ImPULS) for deep brain activation. Nat Commun 15, 4601 (2024). doi: 10.1038/s41467-024-48748-6 [8] Sato T, Shapiro MG, Tsao DY. Ultrasonic neuromodulation causes widespread cortical activation via an indirect auditory mechanism. Neuron 98, 1031–1041.e5 (2018). doi: 10.1016/j.neuron.2018.05.009 [9] Guo HS, Hamilton M, Offutt SJ et al. Ultrasound produces extensive brain activation via a cochlear pathway. Neuron 98, 1020–1030.e4 (2018). doi: 10.1016/j.neuron.2018.04.036 [10] Krauss JK, Lipsman N, Aziz T et al. Technology of deep brain stimulation: current status and future directions. Nat Rev Neurol 17, 75–87 (2021). [11] Chen G, Yu FY, Shi LL et al. High-precision photoacoustic neural modulation uses a non-thermal mechanism. Adv Sci 11, 2403205 (2024). doi: 10.1002/advs.202403205 [12] Du ZY, Chen G, Li YM et al. Photoacoustic: A versatile nongenetic method for high-precision neuromodulation. Acc Chem Res 57, 1595–1607 (2024). doi: 10.1021/acs.accounts.4c00119 [13] Seo D, Neely RM, Shen K et al. Wireless recording in the peripheral nervous system with ultrasonic neural dust. Neuron 91, 529–539 (2016). doi: 10.1016/j.neuron.2016.06.034 [14] Lee T, Baac HW, Li QC et al. Efficient photoacoustic conversion in optical nanomaterials and composites. Adv Opt Mater 6, 1800491 (2018). doi: 10.1002/adom.201800491 [15] Li JP, Yang Y, Chen ZY et al. Self-healing: a new skill unlocked for ultrasound transducer. Nano Energy 68, 104348 (2020). doi: 10.1016/j.nanoen.2019.104348 [16] Ma TG, Wang HZ, Guo LJ. OptoGPT: A foundation model for inverse design in optical multilayer thin film structures. Opto-Electron Adv 7, 240062 (2024). doi: 10.1038/s41467-020-14706-1 [17] Liu HH, Hu DJJ, Sun QZ et al. Specialty optical fibers for advanced sensing applications. Opto-Electron Sci 2, 220025 (2023). doi: 10.1038/nprot.2011.371 [18] Mu MD, Geng HY, Rong KL et al. A limbic circuitry involved in emotional stress-induced grooming. Nat Commun 11, 2261 (2020). doi: 10.1038/s41467-020-16203-x [19] Karatsoreos IN, McEwen BS. Psychobiological allostasis: resistance, resilience and vulnerability. Trends Cogn Sci 15, 576–584 (2011). doi: 10.1016/j.tics.2011.10.005 [20] Kotlęga D, Gołąb-Janowska M, Masztalewicz M et al. The emotional stress and risk of ischemic stroke. Neurol Neurochir Pol 50, 265–270 (2016). doi: 10.1016/j.pjnns.2016.03.006 [21] Gilboa T. Emotional stress-induced seizures: another reflex epilepsy. Epilepsia 53, e29–e32 (2012). [22] Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci 1251, E1–E24 (2012). doi: 10.1111/j.1749-6632.2011.06430.x [23] Hermans EJ, Henckens MJAG, Joëls M et al. Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends Neurosci 37, 304–314 (2014). doi: 10.1016/j.tins.2014.03.006 [24] van Oort J, Tendolkar I, Hermans EJ et al. How the brain connects in response to acute stress: A review at the human brain systems level. Neurosci Biobehav Rev 83, 281–297 (2017). doi: 10.1016/j.neubiorev.2017.10.015 [25] Somerville LH, Wagner DD, Wig GS et al. Interactions between transient and sustained neural signals support the generation and regulation of anxious emotion. Cereb Cortex 23, 49–60 (2013). doi: 10.1093/cercor/bhr373 [26] Sinha R, Lacadie CM, Constable RT et al. Dynamic neural activity during stress signals resilient coping. Proc Natl Acad Sci USA 113, 8837–8842 (2016). doi: 10.1073/pnas.1600965113 [27] Kalinichenko LS, Kornhuber J, Müller CP. Individual differences in inflammatory and oxidative mechanisms of stress-related mood disorders. Front Neuroendocrin 55, 100783 (2019). doi: 10.1016/j.yfrne.2019.100783 [28] Gao JH, Zhang L, Zhu JF et al. Prefrontal cortex hemodynamics and functional connectivity changes during performance working memory tasks in older adults with sleep disorders. Brain Sci 13, 497 (2023). doi: 10.3390/brainsci13030497 [29] Liu TT, Qi CX, Bai WW et al. Behavioral state-dependent oscillatory activity in prefrontal cortex induced by chronic social defeat stress. Front Neurosci 16, 885432 (2022). doi: 10.3389/fnins.2022.885432 [30] Chen JJ, Jin QQ, Li YB et al. Molten salt‐shielded synthesis (MS3) of MXenes in sir. Energy Environ Mater 6, e12328 (2023). doi: 10.1002/eem2.12328 [31] Lu HZ, Wang JH, Li HM et al. Efficient photothermal conversion of MXenes and their application in biomedicine. Mater Chem Front 7, 4372–4399 (2023). doi: 10.1039/D3QM00220A [32] Sayed M, Yu JG, Liu G et al. Non-noble plasmonic metal-based photocatalysts. Chem Rev 122, 10484–10537 (2022). doi: 10.1021/acs.chemrev.1c00473 [33] Cui XM, Ruan QF, Zhuo XL et al. Photothermal nanomaterials: a powerful light-to-heat converter. Chem Rev 123, 6891–6952 (2023). doi: 10.1021/acs.chemrev.3c00159 [34] Brongersma ML, Halas NJ, Nordlander P. Plasmon-induced hot carrier science and technology. Nat Nanotechnol 10, 25–34 (2015). doi: 10.1038/nnano.2014.311 [35] Liu JG, Zhang H, Link S et al. Relaxation of Plasmon-induced hot carriers. ACS Photonics 5, 2584–2595 (2018). doi: 10.1021/acsphotonics.7b00881 [36] Bernardi M, Mustafa J, Neaton JB et al. Theory and computation of hot carriers generated by surface Plasmon polaritons in noble metals. Nat Commun 6, 7044 (2015). doi: 10.1038/ncomms8044 [37] Linic S, Aslam U, Boerigter C et al. Photochemical transformations on plasmonic metal nanoparticles. Nat Mater 14, 567–576 (2015). doi: 10.1038/nmat4281 [38] Baffou G, Quidant R. Thermo-plasmonics: using metallic nanostructures as Nano-sources of heat. Laser Photonics Rev 7, 171–187 (2013). doi: 10.1002/lpor.201200003 [39] Shahzad F, Alhabeb M, Hatter CB et al. Electromagnetic interference shielding with 2D transition metal carbides (MXenes). Science 353, 1137–1140 (2016). doi: 10.1126/science.aag2421 [40] Xing WX, Wang LD, Maslov K et al. Integrated optical- and acoustic-resolution photoacoustic microscopy based on an optical fiber bundle. Opt Lett 38, 52–54 (2013). doi: 10.1364/OL.38.000052 [41] Ansari R, Zhang EZ, Desjardins AE et al. All-optical forward-viewing photoacoustic probe for high-resolution 3D endoscopy. Light Sci Appl 7, 75 (2018). doi: 10.1038/s41377-018-0070-5 [42] Du XY, Li JP, Niu GD et al. Lead halide perovskite for efficient optoacoustic conversion and application toward high-resolution ultrasound imaging. Nat Commun 12, 3348 (2021). doi: 10.1038/s41467-021-23788-4 [43] Yu K, Niu XD, Krook-Magnuson EK et al. Intrinsic functional neuron-type selectivity of transcranial focused ultrasound neuromodulation. Nat Commun 12, 2519 (2021). doi: 10.1038/s41467-021-22743-7 [44] Murphy KR, Farrell JS, Bendig J et al. Optimized ultrasound neuromodulation for non-invasive control of behavior and physiology. Neuron 112, 3252–3266.e5 (2024). doi: 10.1016/j.neuron.2024.07.002 [45] Zhang Z, Ferretti V, Güntan İ et al. Neuronal ensembles sufficient for recovery sleep and the sedative actions of α2 adrenergic agonists. Nat Neurosci 18, 553–561 (2015). doi: 10.1038/nn.3957 [46] Kim J, Kang S, Choi TY et al. Metabotropic glutamate receptor 5 in amygdala target neurons regulates susceptibility to chronic social stress. Biol Psychiatry 92, 104–115 (2022). doi: 10.1016/j.biopsych.2022.01.006 [47] Cattane N, Vernon AC, Borsini A et al. Preclinical animal models of mental illnesses to translate findings from the bench to the bedside: molecular brain mechanisms and peripheral biomarkers associated to early life stress or immune challenges. Eur Neuropsychopharmacol 58, 55–79 (2022). doi: 10.1016/j.euroneuro.2022.02.002 -
Supplementary Information
Supplementary information for Broadband ultrasound generator over fiber-optic tip for in vivo emotional stress modulation
Supplementary Movie S1 
Supplementary Movie S2 
-
Access History
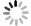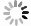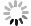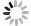
Article Metrics
-
Figure 1.
An implantable fiber-optic photoacoustic neurostimulator. (a) Schematic illustration of the FPE. (b) Broadband ultrasonic excitation of FPE, b1: time-domain and frequency-domain ultrasound response of FPE, b2: lateral distribution of FPE sound field. (c) Schematic diagram of FPE stimulation of medial prefrontal cortex in mice. Blue fiber-optic: fiber photometer for calcium ion recording; light red fiber-optic: FPE photoacoustic neurostimulator.
-
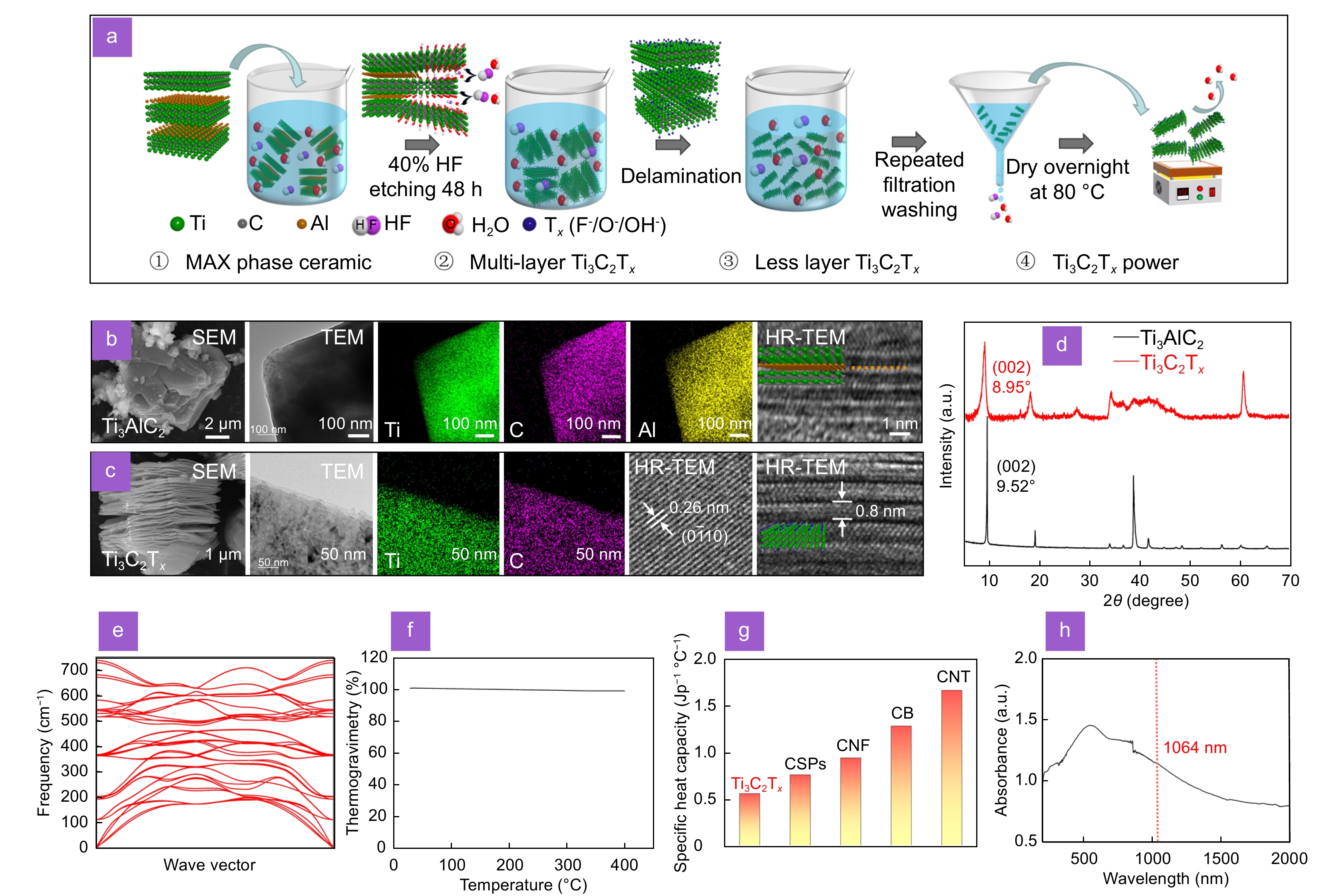
Figure 2.
Characterizations of Ti3C2Tx. (a) Flow chart of preparation of Ti3C2Tx by etching Ti3AlC2 with hydrofluoric acid. SEM and TEM image of Ti3AlC2 (b) and Ti3C2Tx (c) with energy dispersive spectroscopy elemental mappings. (d) XRD patterns of Ti3AlC2 and Ti3C2Tx. (e) The phonon spectrum of Ti3C2Tx through first-principles calculations. (f) The thermogravimetric curve of Ti3C2Tx. (g) Comparison of lattice specific heat capacities of different light-absorbing materials. CSPs: candle-soot carbon nanoparticles; CNF: carbon nanofibers; CB: carbon black; CNT: carbon nanotubes. (h) UV-visible-near-infrared-light absorption spectra of Ti3C2Tx.
-
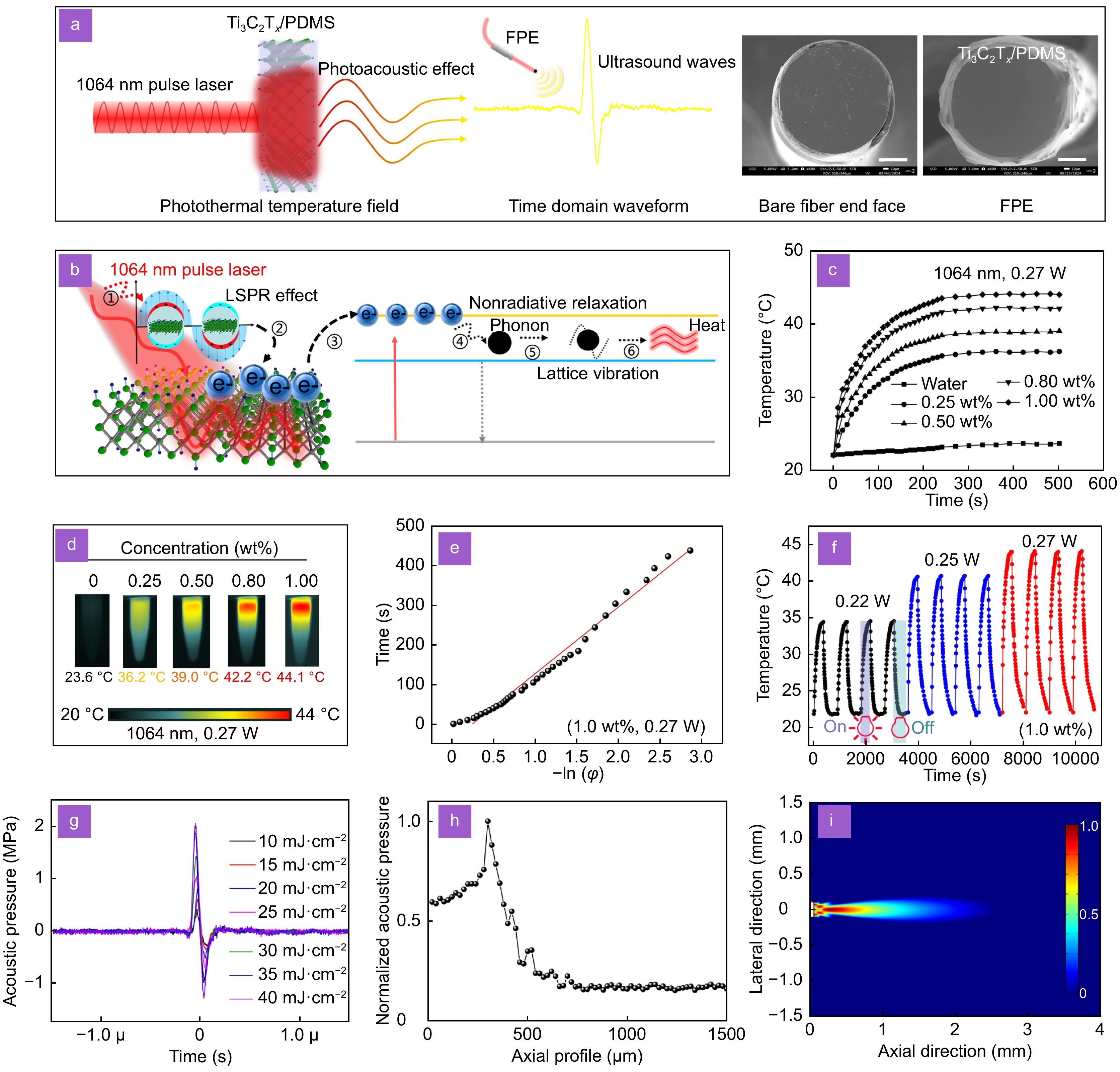
Figure 3.
The photothermal and photoacoustic properties of Ti3C2Tx. (a) A pulse laser periodically regulates the photothermal temperature field of Ti3C2Tx inducing PDMS to excite ultrasound waves, middle image: bare fiber end face, right image: FPE physical image. Scale bar: 50 μm. (b) Photothermal conversion mechanism of Ti3C2Tx, i: pulse laser radiation, ii: LSPR effect, iii: electron transitions, iv: electron-phonon coupling, v: lattice vibration, vi: lattice temperature rise. Temperature change curves (c) and infrared thermal images (d) of Ti3C2Tx concentration: 1.0 wt%, 0.8 wt%, 0.5 wt%, 0.25 wt%, and pure water (
1064 nm, 0.45 g, 0.27 W). (e) Fitting lines of time and −ln(φ) during the cooling process of Ti3C2Tx (1.0 wt%, 0.27 W). (f) Temperature evolution of the Ti3C2Tx in an aqueous dispersion under irradiation with a1064 nm laser at 0.22 W, 0.25 W, and 0.27 W (1.0 wt%). (g) Time domain waveform of FPE excited ultrasound at different pulse laser energy densities. FPE axial acoustic field testing (h) and multiphysics simulation results (i). -
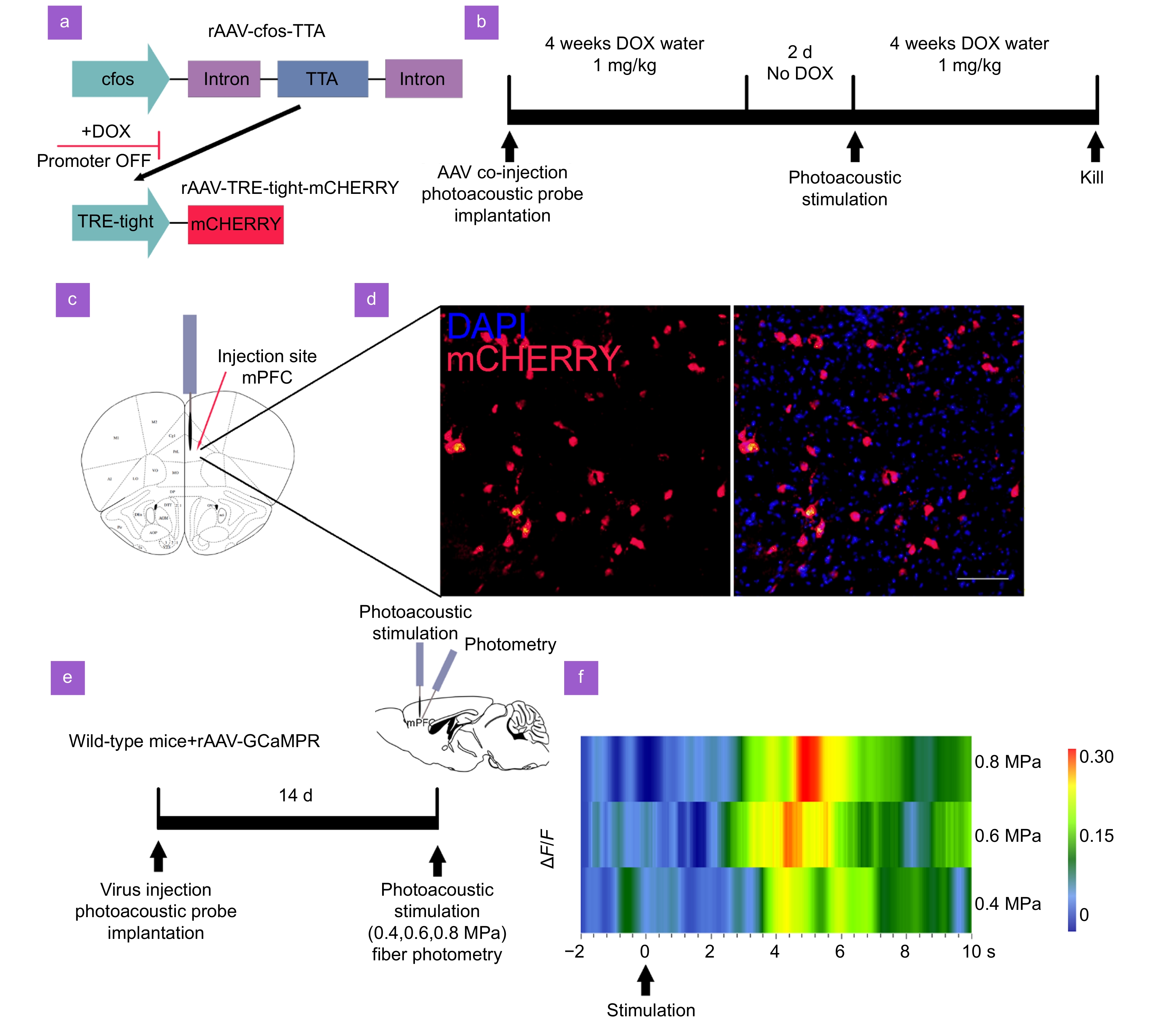
Figure 4.
Photoacoustic stimulation activates mPFC neurons. (a) The AAV vectors. The first component contains the cfos promoter, responsible for driving the expression of the TTA protein. In the presence of DOX, TTA is unable to bind and activate its target promoter, TRE-tight, which is located in the second AAV. Upon removal of DOX, TTA becomes capable of activating mCHERRY expression. Importantly, this activation occurs exclusively in neurons where TTA expression has been induced by the cfos promoter, indicating neural activity. (b) Time line of the experimental procedure. Mice underwent a co-injection of AAV along with the implantation of the FPE. Subsequently, mice were administered DOX water (1 mg/mL) for four weeks, followed by a two-day period without DOX. This was followed by photoacoustic stimulation. Afterwards, mice received another four weeks of DOX in their drinking water. (c) Illustration showing the injection site of the AAV and FPE in the mPFC. (d) Immunofluorescent images displaying cfos expression in mPFC. DAPI (blue) stains the nuclei, and mCHERRY (red) indicates cfos expression. The left image shows cfos expression with photoacoustic stimulation, and the right image depicts the merged cfos and DAPI staining after stimulation. Scale bar: 100 μm. (e) Schematic of the fiber photometry setup for recording calcium signals in wild-type mice injected with rAAV-GCaMP. (f) Heatmap of calcium signal changes (ΔF/F) over time during photoacoustic stimulation (n = 4 mice).
-
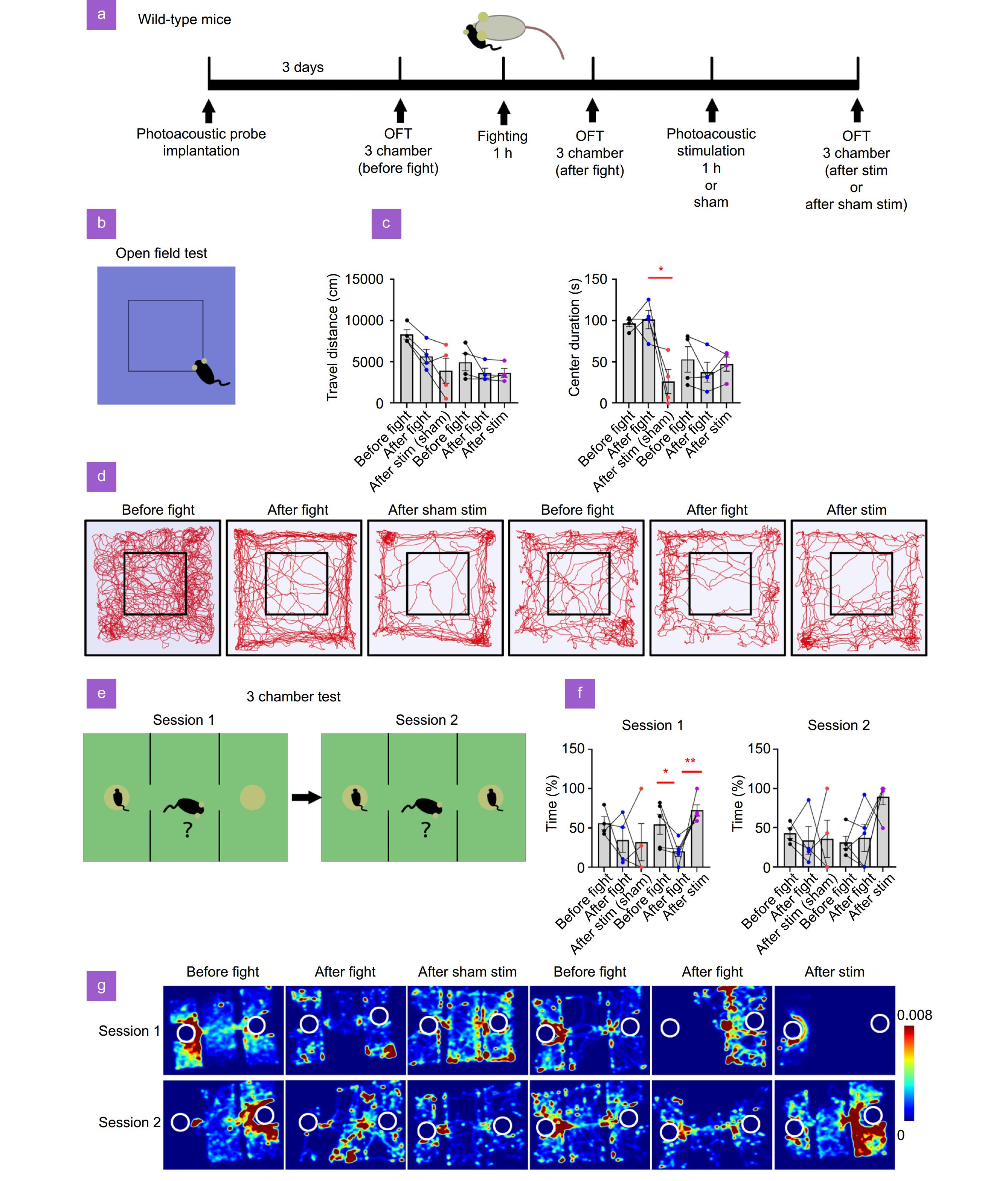
Figure 5.
Photoacoustic stimulation of neurons in mPFC alleviates SDS-induced anxiety and social dysfunction. (a) Time line of the experimental procedure. Mice were implanted with a FPE. After 3 days, the mice underwent OFT and 3 chamber test to assess baseline behavior. Following a 1-hour SDS session, the anxiety was re-evaluated in the OFT and 3-chamber test. Subsequently, mice received either photoacoustic stimulation or sham stimulation for 1 hour before undergoing another OFT and 3 chamber test. (b) Diagram of the OFT setup and the central zone. (c) The total distance traveled and the time spent in the center zone of the OFT arena. (d) Representative trajectory plots from the OFT. (e) Diagram of the 3 chamber test. The diagrams represent session 1 and session 2. (f) Statistical analysis of the percentage of time spent with the left mouse in session 1 and the percentage of time spent with the right mouse in session 2. (g) Heatmaps of mouse movement trajectories. *p<0.05, **p<0.01.

 E-mail Alert
E-mail Alert RSS
RSS

 DownLoad:
DownLoad: